
 �����ڱ���������ر���Һ���ⶨNaOH��Һ��Ũ�ȣ�������������Һ���ζ��ڱ���������Һʱ�����в�����
�����ڱ���������ر���Һ���ⶨNaOH��Һ��Ũ�ȣ�������������Һ���ζ��ڱ���������Һʱ�����в�����| �ζ����� ʵ������ | 1 | 2 | 3 | 4 |
| V����Ʒ��/mL | 20.00 | 20.00 | 20.00 | 20.00 |
| V��NaOH��/mL���������� | 0.10 | 0.30 | 0.00 | 0.20 |
| V��NaOH��/mL���ն����� | 20.08 | 20.30 | 20.80 | 20.22 |
| V��NaOH��/mL�����ģ� | 19.98 | 20.00 | 20.80 | 20.02 |
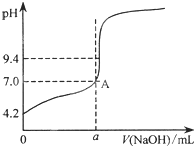
���� ��1�������к͵ζ��м�©��ϴ�ӡ���ϴ��װҺ��ȡ����Һ���ζ��Ȳ��������жϣ��ζ�ʱ���õζ��ܴ���Һȡ20mL����Һ������ƿ�У����ݵζ��յ��pHҪ��ָʾ���ı�ɫ��Χ֮��ȷ��ָʾ����
��2����3�����ݺ������������ϴ�����������Һ�����ȡ����3��ƽ��ֵ��
��3���ڱ���������ر���Һʢ������ʽ�ζ����У����ٷ�Һ���ɸ�����ʽ�ζ����е����ݣ�
��4���ζ�ǰ��������ˮϴ����ʽ�ζ��ܣ�Ȼ��Ӵ��ⶨ������������Һ�ζ�������Һ��ϡ�ͣ�
��� �⣺��1���к͵ζ����ռ�©��ϴ�ӡ���ϴ��װҺ��ȡ����Һ���ζ���˳������������˳��Ϊ�ݢڢ٢ۢܢޣ��ζ�ʱ������ʽ�ζ��ܴ���Һȡ20mL�ڱ���������ط�����ƿ�У��ڱ����������Ϊ���ᣬ�յ�ʱ��Һ��pHԼΪ9.1���ζ��յ��pHҪ��ָʾ���ı�ɫ��Χ֮�ڣ�����ѡ�÷�̪��ָʾ����
�ʴ𰸣��ݢڢ٢ۢܢޣ���ʽ�ζ��ܣ���̪��
��2����3�����ݺ������������ϴ�����������Һ�����ȡ����3��ƽ��ֵΪ��$\frac{17.98+20.00+20.02}{3}$mL=20.00mL��
�ʴ�Ϊ������������3�����ݺ������������ϴ�Ӧ���ã�����
��3���ڱ���������ر���Һʢ������ʽ�ζ����У�����ζ����ڲ������ݣ��������ݵIJ������ٷ�Һ���ʴ�Ϊ�����ٷ�Һ��
��4���ζ�ǰ��������ˮϴ����ʽ�ζ��ܣ�Ȼ��Ӵ��ⶨ������������Һ�ζ�������Һ��ϡ�ͣ�����Һ��Ũ��ƫС���ʴ�Ϊ��ƫС��
���� ���⿼�����к͵ζ��������������Լ����㣬�ѶȲ��������к͵ζ���ԭ���ǽ���ؼ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ǵķ���ʽ��C6H12O6 | |
| B�� | �������Ƕ��ǻ�ȩ���������ȩ�Ͷ�Ԫ�������� | |
| C�� | �����������ȩ�� | |
| D�� | �����ǿ���ͨ����ɫֲ�������úϳ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | �ζ�ǰ���ζ��ܼ��첿�������ݣ��ζ���������ʧ | |
| B�� | ��ƿմ������ˮ | |
| C�� | �Լ�����ָʾ�� | |
| D�� | �ζ�ǰ���ӵζ��ܶ������ζ���ƽ�ӿ̶ȶ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����AgCl��������Һ�м���NaC1���壬c��Ag+����С | |
| B�� | ������100mL pH=1.3��Ba��OH��2��Һ��OH-�����ʵ���Ϊ0.02 mol | |
| C�� | ϡ��0.1 mol/L��NH3•H2O��Һ����Һ����������Ũ�Ⱦ���С | |
| D�� | ��Һ��ˮ�����c��H+����ˮ�������c��OH-���ij˻�һ������10-14 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
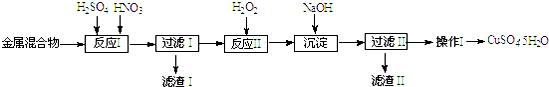
| ������ | Fe3+ | Fe2+ | Cu2+ |
| ��ʼ���� | 1.5 | 6.4 | 4.2 |
| ��ȫ���� | 3.2 | 8.9 | 6.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ij��ѧʵ��С����0.2000 mol/L������KMnO4��Һ�ⶨ���ᾧ��Ĵ��ȣ����ᾧ�廯ѧʽΪH2C2O4•2H2O�����ʲ���KMnO4��Ӧ����ʵ�鲽�����£�
ij��ѧʵ��С����0.2000 mol/L������KMnO4��Һ�ⶨ���ᾧ��Ĵ��ȣ����ᾧ�廯ѧʽΪH2C2O4•2H2O�����ʲ���KMnO4��Ӧ����ʵ�鲽�����£�| �ζ����� | ������Һ��� | ����KMnO4��Һ��� | |
| �ζ�ǰ����/m L | �ζ������/m L | ||
| ��һ�� | 25.00 | 0.20 | 20.58 |
| �ڶ��� | 25.00 | 4.00 | 24.40 |
| ������ | 25.00 | 2.38 | a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ���嶡�����ӣ�
���嶡�����ӣ�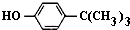 ����ҵ��;�㷺�����������������Է�ȩ��֬���ȶ��������ϵȣ�ʵ�����Ա��ӡ��嶡����[��CH3��3CCl]��Ϊԭ���Ʊ����嶡�����ӣ�ʵ�鲽�����£�
����ҵ��;�㷺�����������������Է�ȩ��֬���ȶ��������ϵȣ�ʵ�����Ա��ӡ��嶡����[��CH3��3CCl]��Ϊԭ���Ʊ����嶡�����ӣ�ʵ�鲽�����£� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��ʼ���ʵ���Ũ�ȣ�mol/L�� | 1.5 | 1 | 0 |
| 2sĩ���ʵ���Ũ�ȣ�mol/L�� | 0.9 | 0.8 | 0.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com