
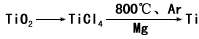

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

(1)Ti��ԭ������Ϊ22��Tiλ��Ԫ�����ڱ��е�_________���ڣ���_________�塣
(2)����ټ�Fe��Ŀ����___________________________��
�������ȴ��Ŀ����______________________________��
(3)�����Ʊ�TiO2�Ĺ����У��������õĸ�������__________�����dzɱ��ͷ����ۺ��������أ���Һ��Ӧ����__________������
(4)�ɽ��ʯ(TiO2)��ȡ����Ti���漰���IJ���Ϊ
TiO2��TiCl4![]() Ti
Ti
��֪����C(s)+O2(g)![]() CO2(g);��H=-393.5 KJ��mol-1
CO2(g);��H=-393.5 KJ��mol-1
��2CO(g)+O2(g)![]() 2CO2(g);��H=-566 KJ��mol-1
2CO2(g);��H=-566 KJ��mol-1
��TiO2(s)+2Cl2(g)![]() TiCl4(s)+O2(g);��H=+141 KJ��mol-1
TiCl4(s)+O2(g);��H=+141 KJ��mol-1
��TiO2(g)+2Cl2(g)+2C(s)![]() TiCl4(s)+2CO(g)�Ħ�H=________________��
TiCl4(s)+2CO(g)�Ħ�H=________________��
��ӦTiCl4+2Mg![]() 2MgCl2+Ti��Ar�����н��е�������________________��
2MgCl2+Ti��Ar������������________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

(1)Ti��ԭ������Ϊ22,Tiλ��Ԫ�����ڱ��е�_________���ڣ���_________�塣
(2)����ټ�Fe��Ŀ����___________________________��
�������ȴ��Ŀ��________________________________��
(3)�����Ʊ�TiO2�Ĺ����У��������õĸ�������_________�����dzɱ��ͷ����ۺ��������أ���Һ��Ӧ����_________������
(4)�ɽ��ʯ(TiO2)��ȡ����Ti���漰���IJ���Ϊ��
TiO2![]() TiCl4
TiCl4![]() Ti
Ti
��֪����C(s)+O2(g)====CO2(g)����H=-393.5 kJ��mol-1
��2CO(g)+O2(g)====2CO2(g)����H=-566 kJ��mol-1
��TiO2(s)+2Cl2(g)====TiCl4(s)+O2(g)����H=+141 kJ��mol-1
��TiO2(s)+2Cl2(g)+2C(s)====TiCl4(s)+2CO(g)�Ħ�H=__________��
��ӦTiCl4+2Mg====2MgCl2+Ti��Ar�����н��е�������____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��6�֣���1��1.00L1.00mol/LH2SO4��Һ��2.00L1.00mol/LNaOH��Һ��ȫ��Ӧ���ų�114.6kJ����������ʾ���к��ȵ��Ȼ�ѧ����ʽΪ ��
��2���ɽ��ʯ(TiO2)��ȡ����Ti���漰���IJ���Ϊ��
![]()
��֪����C(s)+ O2(g) = CO2(g)����H=-393.5kJ/mol
��2CO(g) + O2(g) = 2CO2(g)����H=-566kJ/mol
��TiO2(s) + 2Cl2(g)= TiCl4(s)+O2(g)����H=+141kJ/mol
��a��̼�������в���ȫȼ������CO���Ȼ�ѧ����ʽΪ
��
b��TiO2(s) + 2Cl2(g) + 2C(s) = TiCl4(s) + 2CO(g)�Ħ�H=________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010������ʡ��Ϫһ�и߶���ѧ����ĩ���Ի�ѧ���� ���ͣ������
��6�֣���1��1.00L1.00mol/LH2SO4��Һ��2.00L1.00mol/LNaOH��Һ��ȫ��Ӧ���ų�114.6kJ����������ʾ���к��ȵ��Ȼ�ѧ����ʽΪ ��
��2���ɽ��ʯ(TiO2)��ȡ����Ti���漰���IJ���Ϊ��
��֪����C(s) + O2(g) = CO2(g)����H=-393.5kJ/mol
��2CO(g) + O2(g) = 2CO2(g)����H=-566kJ/mol
��TiO2(s) + 2Cl2(g) = TiCl4(s)+O2(g)����H=+141kJ/mol
2Cl2(g) = TiCl4(s)+O2(g)����H=+141kJ/mol
��a��̼�������в���ȫȼ������CO���Ȼ�ѧ����ʽΪ
��
b��TiO2(s) + 2Cl2(g) + 2C(s) = TiCl4(s) + 2CO(g)�Ħ�H=________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010������ʡ�߶���ѧ����ĩ���Ի�ѧ���� ���ͣ������
��6�֣���1��1.00L1.00mol/LH2SO4��Һ��2.00L1.00mol/LNaOH��Һ��ȫ��Ӧ���ų�114.6kJ����������ʾ���к��ȵ��Ȼ�ѧ����ʽΪ ��
��2���ɽ��ʯ(TiO2)��ȡ����Ti���漰���IJ���Ϊ��

��֪����C(s) + O2(g) = CO2(g)����H=-393.5kJ/mol
��2CO(g) + O2(g) = 2CO2(g)����H=-566kJ/mol
��TiO2(s) + 2Cl2(g) = TiCl4(s)+O2(g)����H=+141kJ/mol
��a��̼�������в���ȫȼ������CO���Ȼ�ѧ����ʽΪ
��
b��TiO2(s) + 2Cl2(g) + 2C(s) = TiCl4(s) + 2CO(g)�Ħ�H=________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com