



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 A”¢B”¢C”¢D¾łĪŖ³£¼ūĪļÖŹ£¬Ļą»„Ö®¼äµÄ¹ŲĻµČēĶ¼ĖłŹ¾£®”°-”±±ķŹ¾Į½ÖÖĪļÖŹ¼äÄÜ·¢Éś·“Ó¦£¬”°”ś”±±ķŹ¾ĪļÖŹ¼äµÄ×Ŗ»Æ¹ŲĻµ£¬²æ·Ö·“Ó¦Īļ»ņÉś³ÉĪļŅŌ¼°·“Ó¦Ģõ¼žŅŃĀŌČ„£©£®
A”¢B”¢C”¢D¾łĪŖ³£¼ūĪļÖŹ£¬Ļą»„Ö®¼äµÄ¹ŲĻµČēĶ¼ĖłŹ¾£®”°-”±±ķŹ¾Į½ÖÖĪļÖŹ¼äÄÜ·¢Éś·“Ó¦£¬”°”ś”±±ķŹ¾ĪļÖŹ¼äµÄ×Ŗ»Æ¹ŲĻµ£¬²æ·Ö·“Ó¦Īļ»ņÉś³ÉĪļŅŌ¼°·“Ó¦Ģõ¼žŅŃĀŌČ„£©£®

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 A”¢B”¢C”¢D¾łĪŖ֊ѧ»Æѧ֊³£¼ūµÄĪļÖŹ£¬ĖüĆĒÖ®¼äµÄ×Ŗ»Æ¹ŲĻµČēĻĀĶ¼£Ø²æ·Ö²śĪļŅŃĀŌČ„£©£ŗŹŌ»Ų“š£ŗ
A”¢B”¢C”¢D¾łĪŖ֊ѧ»Æѧ֊³£¼ūµÄĪļÖŹ£¬ĖüĆĒÖ®¼äµÄ×Ŗ»Æ¹ŲĻµČēĻĀĶ¼£Ø²æ·Ö²śĪļŅŃĀŌČ„£©£ŗŹŌ»Ų“š£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 A”¢B”¢C”¢D¾łĪŖ¶ĢÖÜĘŚŌŖĖŲ£¬AŗĶBŹĒĶ¬ÖÜĘŚĻąĮŚµÄĮ½ÖÖŌŖĖŲ£¬AŗĶCŹĒĶ¬Ö÷×åĻąĮŚµÄĮ½ÖÖŌŖĖŲ£ŗA”¢B”¢CČżÖÖŌŖĖŲµÄŌ×ÓŠņŹżÖ®ŗĶĪŖ31£»DŌŖĖŲÓėA”¢B”¢CČżÖÖŌŖĖŲ¼Č²»ŹĒĶ¬ÖÜĘŚ£¬Ņ²²»Ķ¬Ö÷×壮Ēė»Ų“š£ŗ
A”¢B”¢C”¢D¾łĪŖ¶ĢÖÜĘŚŌŖĖŲ£¬AŗĶBŹĒĶ¬ÖÜĘŚĻąĮŚµÄĮ½ÖÖŌŖĖŲ£¬AŗĶCŹĒĶ¬Ö÷×åĻąĮŚµÄĮ½ÖÖŌŖĖŲ£ŗA”¢B”¢CČżÖÖŌŖĖŲµÄŌ×ÓŠņŹżÖ®ŗĶĪŖ31£»DŌŖĖŲÓėA”¢B”¢CČżÖÖŌŖĖŲ¼Č²»ŹĒĶ¬ÖÜĘŚ£¬Ņ²²»Ķ¬Ö÷×壮Ēė»Ų“š£ŗ

 NH3?H2O+H+
NH3?H2O+H+ NH3?H2O+H+
NH3?H2O+H+ 2NH3
2NH3 2NH3
2NH3

| 4(m-n) |
| 5 |
| 4(m-n) |
| 5 |
| 4n+m |
| 5 |
| 4n+m |
| 5 |
| 4m+3n |
| 5 |
| 4m+3n |
| 5 |
| m-3n |
| 5 |
| m-3n |
| 5 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
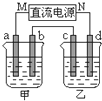 ČēĶ¼ĖłŹ¾µÄ×°ÖĆÖŠ£¬¼×”¢ŅŅĮ½ÉÕ±ÖŠ·Ö±šŹ¢·ÅÓŠ×ćĮæµÄCuSO4ČÜŅŗŗĶ100g 10.00%µÄK2SO4ČÜŅŗ£¬a”¢b”¢c”¢d¾łĪŖŹÆÄ«µē¼«£®½ÓĶصēŌ“Ņ»¶ĪŹ±¼äŗ󣬲āµĆŅŅÖŠK2SO4ČÜŅŗÅضČĪŖ10.47%£¬¼×ÖŠaµē¼«µÄÖŹĮæŌö¼Ó£®ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©
ČēĶ¼ĖłŹ¾µÄ×°ÖĆÖŠ£¬¼×”¢ŅŅĮ½ÉÕ±ÖŠ·Ö±šŹ¢·ÅÓŠ×ćĮæµÄCuSO4ČÜŅŗŗĶ100g 10.00%µÄK2SO4ČÜŅŗ£¬a”¢b”¢c”¢d¾łĪŖŹÆÄ«µē¼«£®½ÓĶصēŌ“Ņ»¶ĪŹ±¼äŗ󣬲āµĆŅŅÖŠK2SO4ČÜŅŗÅضČĪŖ10.47%£¬¼×ÖŠaµē¼«µÄÖŹĮæŌö¼Ó£®ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©| A”¢¼×”¢ŅŅČÜŅŗµÄpH¾ł¼õŠ” | B”¢µē¼«bÉĻÉś³ÉĘųĢåµÄĢå»żŌ¼ĪŖ2.8L£Ø±ź×¼×“æöĻĀ£© | C”¢µē¼«dÉĻ·¢ÉśµÄ·“Ó¦ĪŖ£ŗ2H2O+2e-?H2”ü+2OH- | D”¢ÓūŹ¹¼×ÖŠµÄČÜŅŗ»Öø“ÖĮŌĄ“µÄÅØ¶Č£¬æɼÓČė24.5gµÄCu£ØOH£©2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 £®¼×ŗĶ±ūæÉŠĪ³ÉĮ½ÖÖ»ÆŗĻĪļXŗĶY£¬XŗĶĖ®·“Ó¦ŗóÉś³ÉŅ»ÖÖ¾ßÓŠ»¹ŌŠŌµÄ¶žŌŖĖįM£®1molŅŅÓė×ćĮæ±ūæÉ»ÆŗĻÉś³ÉZ£¬ĖłµĆµÄZÓėČČĖ®·“Ó¦µÄ²śĪļWŠčÓĆ12mol KOH²ÅÄÜĶźČ«ÖŠŗĶ£®ŅŅŌŚ×ćĮæ¶”ÖŠČ¼ÉÕÉś³É»ÆŗĻĪļN£¬NŗĶĖ®·“Ӧɜ³ÉWŗĶDŌŖĖŲµÄĘųĢ¬Ēā»ÆĪļ£®ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
£®¼×ŗĶ±ūæÉŠĪ³ÉĮ½ÖÖ»ÆŗĻĪļXŗĶY£¬XŗĶĖ®·“Ó¦ŗóÉś³ÉŅ»ÖÖ¾ßÓŠ»¹ŌŠŌµÄ¶žŌŖĖįM£®1molŅŅÓė×ćĮæ±ūæÉ»ÆŗĻÉś³ÉZ£¬ĖłµĆµÄZÓėČČĖ®·“Ó¦µÄ²śĪļWŠčÓĆ12mol KOH²ÅÄÜĶźČ«ÖŠŗĶ£®ŅŅŌŚ×ćĮæ¶”ÖŠČ¼ÉÕÉś³É»ÆŗĻĪļN£¬NŗĶĖ®·“Ӧɜ³ÉWŗĶDŌŖĖŲµÄĘųĢ¬Ēā»ÆĪļ£®ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com