
�����dz����ĵ绯ѧװ��ͼ��
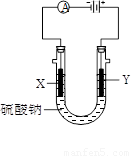
ͼ1 ͼ2 ͼ3
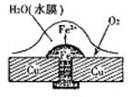
��ش��������⣺��1��ͼ1��ͭ������í�����������⣬��Ϊ ��ʴ������ʴ�Ľ����� ��ԭ��ص��ܷ�Ӧ����ʽ�� ��
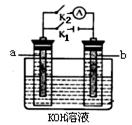
 ��2��ͼ2��a��b�Ƕ��ʯī�缫���Ͽ�K2���պ�K1һ��ʱ�䣬�۲쵽��ֻ�������ڶ������ݽ��缫��Χ��b���ϵĵ缫��ӦʽΪ
��OH-��
����a��b�����ƶ���Ȼ��Ͽ�K1���պ�K2���۲쵽������A��ָ����ƫת��b���ϵĵ缫��ӦʽΪ
��OH-�� ����a��b�����ƶ���
��2��ͼ2��a��b�Ƕ��ʯī�缫���Ͽ�K2���պ�K1һ��ʱ�䣬�۲쵽��ֻ�������ڶ������ݽ��缫��Χ��b���ϵĵ缫��ӦʽΪ
��OH-��
����a��b�����ƶ���Ȼ��Ͽ�K1���պ�K2���۲쵽������A��ָ����ƫת��b���ϵĵ缫��ӦʽΪ
��OH-�� ����a��b�����ƶ���
��3��ͼ3�У�X��Y��Ϊʯī�缫����U������ֱ����һ����ɫʯ����Һ����ͨ���X������______ɫ��Y������_______ɫ��
��1�� ���� Fe 2Fe+O2+2H2O=2Fe(OH)2
��2�� 2H++2e��=H2 a H2+2OH����2e��=2H2O b ��3���� ��
����������1�������������Ļ�����������Һ�����Ժ���������������ʴ������ͭ���ã���ʧȥ���ӱ���ʴ�����������ܽ���ˮ�е������õ����ӣ�������ԭ��Ӧ��
��2���Ͽ�K2���պ�K1�ɵ��أ��缫����ʯī���������������Һ���൱�ڵ��ˮ��b���Դ�ĸ����������������������ӷŵ�������������Һ�е�OH���������Ϸŵ磬����������������a���ƶ����Ͽ�K1���պ�K2���۲쵽������A��ָ����ƫת��˵����ʱ����ԭ��أ���b��Χ���������ڣ��������������Һ�е��������������ƶ������������ƶ���
��3��ʯī�缫�����������Һ���൱�ڵ��ˮ��Y���Դ����������������������Һ�е�OH���ŵ磬����������X����������Һ�е������ӷŵ��������������X��Χ��Һ�Լ��ԣ�Y��Χ��Һ�����ԡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�ٵ��CuCl2��Һװ�� �ڵ��ͭʵ��װ��

������ȼ�ϵ��ʾ��ͼ �����ӽ���Ĥ�����ԭ��ʾ��ͼ
(1)д��װ�â��з�����Ӧ���ܻ�ѧ����ʽ__________________________________��
(2)װ�â���ͭƬΪ______������ͭƬ������Ʒ��������ȣ����һ��ʱ���·����2 mol����ת�ƣ���ʱͭƬ������Ʒ��������Ϊ____________g��
(3)װ�â���b��Ϊ______�����ü��ĵ缫��Ӧ����ʽΪ_________________��
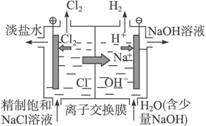
(4)װ�â�Ϊ��ҵ�ϵ��ʳ��ˮ��ȡNaOH��ԭ��ʾ��ͼ�����������ӽ���Ĥ�����۸��������Һ������ң������Ҽ��뾫�Ƶı���ʳ��ˮ�������Ҽ��봿ˮ����һ������NaOH����
����������Ĵ�ˮ�м���NaOH��ԭ��__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����dz����ĵ绯ѧװ��ͼ���٢ۢ��о�Ϊ���Ե缫������˵����ȷ����

A. װ�â��е��һ��ʱ�����Һ��PH���䡣
B. װ�â���b��Ϊ�������ü��ĵ缫��Ӧ����ʽΪO2+4H++ 4e-=2H2O
C. װ�â���ͭƬΪ��������ͭƬ������Ʒ��������ȣ����һ��ʱ���·����2mol
����ת�ƣ���ʱͭƬ������Ʒ��������Ϊ128g
D. װ�â��������ӽ���Ĥ��ÿ��1mol Na+ʱ���������ϲ���11.2L H2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����dz����ĵ绯ѧװ��ͼ��

ͼ1 ͼ2 ͼ3
��ش��������⣺��1��ͼ1��ͭ������í�����������⣬��Ϊ ��ʴ������ʴ�Ľ����� ��ԭ��ص��ܷ�Ӧ����ʽ�� ��
![]() ��2��ͼ2��a��b�Ƕ��ʯī�缫���Ͽ�K2���պ�K1һ��ʱ�䣬�۲쵽��ֻ�������ڶ������ݽ��缫��Χ��b���ϵĵ缫��ӦʽΪ ��OH-�� ����a��b�����ƶ���Ȼ��Ͽ�K1���պ�K2���۲쵽������A��ָ����ƫת��b���ϵĵ缫��ӦʽΪ ��OH-�� ����a��b�����ƶ���
��2��ͼ2��a��b�Ƕ��ʯī�缫���Ͽ�K2���պ�K1һ��ʱ�䣬�۲쵽��ֻ�������ڶ������ݽ��缫��Χ��b���ϵĵ缫��ӦʽΪ ��OH-�� ����a��b�����ƶ���Ȼ��Ͽ�K1���պ�K2���۲쵽������A��ָ����ƫת��b���ϵĵ缫��ӦʽΪ ��OH-�� ����a��b�����ƶ���
��3��ͼ3�У�X��Y��Ϊʯī�缫����U������ֱ����һ����ɫʯ����Һ����ͨ���X������______ɫ��Y������_______ɫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ������ѧ��һ��ѧ�ڵ�һ�ν��Բ��Ի�ѧ�Ծ����������� ���ͣ������
�����dz����ĵ绯ѧװ��ͼ��


ͼ1 ͼ2 ͼ3
��ش��������⣺��1��ͼ1��ͭ������í�����������⣬��Ϊ ��ʴ������ʴ�Ľ����� ��ԭ��ص��ܷ�Ӧ����ʽ�� �� ��2��ͼ2��a��b�Ƕ��ʯī�缫���Ͽ�K2���պ�K1һ��ʱ�䣬�۲쵽��ֻ�������ڶ������ݽ��缫��Χ��b���ϵĵ缫��ӦʽΪ ��OH-�� ����a��b�����ƶ���Ȼ��Ͽ�K1���պ�K2���۲쵽������A��ָ����ƫת��b���ϵĵ缫��ӦʽΪ ��OH-�� ����a��b�����ƶ���
��2��ͼ2��a��b�Ƕ��ʯī�缫���Ͽ�K2���պ�K1һ��ʱ�䣬�۲쵽��ֻ�������ڶ������ݽ��缫��Χ��b���ϵĵ缫��ӦʽΪ ��OH-�� ����a��b�����ƶ���Ȼ��Ͽ�K1���պ�K2���۲쵽������A��ָ����ƫת��b���ϵĵ缫��ӦʽΪ ��OH-�� ����a��b�����ƶ���
��3��ͼ3�У�X��Y��Ϊʯī�缫����U������ֱ����һ����ɫʯ����Һ����ͨ���X������______ɫ��Y������_______ɫ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com