
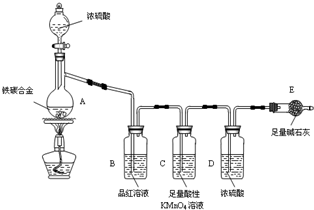
| 12b |
| 44 |
| 3b |
| 11 |
| 3b |
| 11 |
| 11a-3b |
| 11a |
| 11a-3b |
| 11a |


 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д� ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д� �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��1 mol H2SO4��1 mol Ba��OH��2��ȫ�к����ų�������Ϊ�к��� |
| B���кͷ�Ӧ���Ƿ��ȷ�Ӧ��һ�������¶�����̼��̼��ԭ�����ȷ�Ӧ |
| C����101 kPaʱ��1 mol̼ȼ�����ų�������һ����̼��ȼ���� |
| D��COȼ�������ȷ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
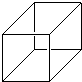 �������顱��һ���ºϳɵ����������Ϊ��������ṹ����̼�Ǽܽṹ��ͼ��ʾ��
�������顱��һ���ºϳɵ����������Ϊ��������ṹ����̼�Ǽܽṹ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
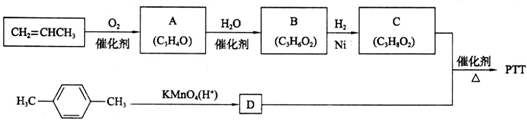
| CI2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
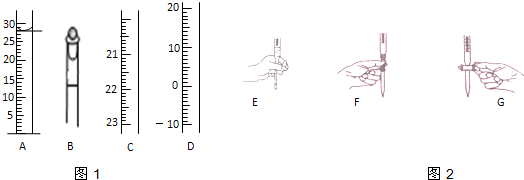
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��� | ʵ������ | ʵ��Ŀ�� |
| A | �����£�ʹ��pH�ƲⶨŨ�Ⱦ�Ϊ0.1mol/L NaClO��Һ��CH3COONa��Һ��pH | �Ƚ�HClO��CH3COOH������ǿ�� |
| B | �����£�����֧װ��ͬ���ͬŨ��H2O2��Һ���Թ��У��ֱ����3��ͬŨ�ȵ� CuSO4��FeSO4��Һ | �Ƚ�CuSO4��FeSO4��Ϊ������H2O2�ֽ����ʵ�Ӱ�� |
| C | ��0.1mol/L AgNO3��Һ�еμ�0.1mol/L NaCl��Һ���������а�ɫ�������ɣ��������е���0.1mol/L KI��Һ���۲������ɫ�仯 | �Ƚ�AgCl��AgI���ܽ����Դ�С�� |
| D | ��������FeCl3��MgCl2������Һ�м�������Mg��OH��2�����Ȳ����裬���� | ��ȥMgCl2������Һ�к��е�����FeCl3 |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��2��7 | B��3��5 |
| C��1��4 | D��4��3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
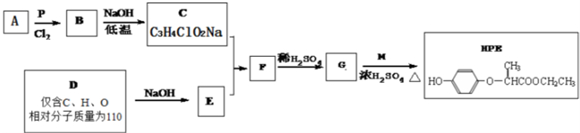
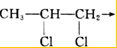
| P |
| Cl2 |
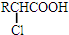
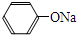 +RCl��
+RCl��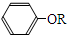 +NaCl
+NaCl�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
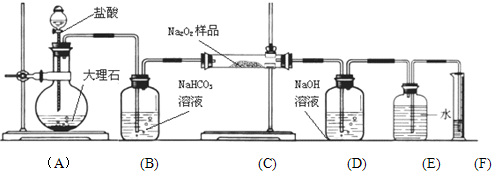
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com