

| ŹµŃé²Ł×÷ | Ō¤ĘŚĻÖĻóÓė½įĀŪ |
| ²½Öč1£ŗȔɣĮæ“ż²āŅŗ·ÅČėŹŌ¹ÜÖŠ£¬µĪ¼Ó¹żĮælmol/LĀČ»Æ±µČÜŅŗ”£¾²ÖĆŅ»¶ĪŹ±¼äŗó£¬µĆµ½ĀĖŅŗAŗĶ¹ĢĢåB”£ | |
| ²½Öč2£ŗĶł¹ĢĢåBÖŠ¼ÓČėÕōĮóĖ®Ļ“µÓ³Įµķ£¬¾²ÖĆŗóʜȄÉĻ²ćĒåŅŗ£¬Ļņ¹ĢĢåµĪČė2µĪ£Ø»ņÉŁĮæ£©Ę·ŗģ£¬ŌŁ | ČōĘ·ŗģĶŹÉ«£Ø»ņÓŠĘųÅŻ£©£¬Ōņ |
| ²½Öč3£ŗ | Čō Ōņ £» ·ńŌņ ”£ |
| ŹµŃé²Ł×÷ | Ō¤ĘŚĻÖĻóÓė½įĀŪ |
| ²½Öč2£ŗŌŁµĪČė¹żĮæ2mol/LŃĪĖį£Ø1·Ö£©£¬Õńµ“£Ø1·Ö£© | ²śĪļÖŠ“ęŌŚNa2SO3£Ø1·Ö£© |
| ²½Öč3£ŗÓĆŹŌ¹ÜČ”ŹŹĮæĀĖŅŗA£Ø1·Ö£©£¬ĻņĘäÖŠ ¼ÓČė¹żĮælmol/LĒāŃõ»Æ±µČÜŅŗ£Ø»ņµĪČė2µĪĘ·ŗģ£¬ŌŁµĪČė¹żĮæ2mol/LŃĪĖį”££©£¬Õńµ“£Ø1·Ö£© | Čō³öĻÖ»ė×Ē£Ø»ņŗģÉ«ĶŹČ„£©£¬Ōņ²śĪļÖŠ“ęŌŚNaHSO3£Ø1·Ö£©£»·ńŌņ²»“ęŌŚNaHSO3£Ø1·Ö£©”£ |
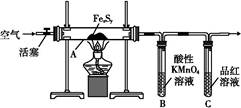
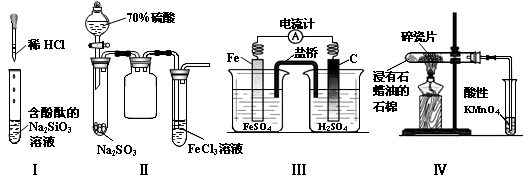
 CuSO4+SO2”ü+2H2O£¬SO2²»½öŅ×ČÜÓŚĖ®£¬¶ųĒŅŅ×ČÜӌװÖƱūÖŠµÄNaOHČÜŅŗ£¬Ņņ“Ė±ūÖŠČÜŅŗŅ×µ¹Īü½ųČė¼××°ÖĆÄŚµÄÅØĮņĖįÖŠ£¬Ņ×Ōģ³É°²Č«ŹĀ¹Ź£¬ĖłŅŌ×°ÖĆŅŅµÄ×÷ÓĆ¾ĶŹĒ·ĄÖ¹±ūÖŠŅŗĢåµ¹ĪüČė×°ÖĆ¼×ÖŠ£Ø»ņĘš»ŗ³åĘæ»ņ°²Č«ĘæµÄ×÷ÓĆ£©£»£Ø2£©¶”×°ÖĆÖŠKMnO4×÷ĒæŃõ»Æ¼Į£¬SO2×÷»¹Ō¼Į£¬ĆĢŌŖĖŲÓÉ+7¼Ū½µĪŖ+2¼Ū£¬ĮņŌŖĖŲÓÉ+4¼ŪÉżĪŖ+6¼Ū£¬øł¾Ż»ÆŗĻ¼ŪÉż½µ×ÜŹżĻąµČ”¢Ō×ÓøöŹżŹŲŗćæÉÖŖ£¬2KMnO4+5SO2+2H2O=2MnSO4+K2SO4+2H2SO4£¬»ņÕß5SO2£«2MnO4££«2H2O=5SO42££«2Mn2+£«4H+£»£Ø3£©ĀČĖ®ŹĒĒæŃõ»Æ¼Į£¬æÉŅŌ½«SO2Ńõ»ÆĪŖSO42££¬¼“SO2+Cl2+2H2O=H2SO4+2HCl£¬ŌņSO2ŹĒ»¹Ō¼Į£¬ĻŌ»¹ŌŠŌ£»£Ø4£©ČōSO2²»×ć£¬±ūÖŠ·“Ó¦ĪŖSO2+2NaOH=Na2SO3+H2O£»ČōSO2¹żĮ棬±ūÖŠĻČ·¢ÉśµÄ·“Ó¦ĪŖSO2+2NaOH=Na2SO3+H2O£¬ŗó·¢ÉśµÄ·“Ó¦ĪŖNa2SO3+H2O+SO2=2NaHSO3£¬ÓÉ“ĖĶʶĻ±ūÖŠSO2¹żĮ棬ŌņµĪČėĘ·ŗģČÜŅŗ»įĶŹÉ«£¬ŅņĪŖ¶žŃõ»ÆĮņ¾ßÓŠĘư׊Ō£»£Ø5£©Na2SO3ÓėBaCl2Ņ×·“Ӧɜ³ÉBaSO3³Įµķ£¬¶ųNaHSO3ÓėBaCl2²»ÄÜ·“Ó¦£»BaSO3Ņ×ČÜÓŚŃĪĖį£¬²¢·Å³öÄÜŹ¹Ę·ŗģČÜŅŗĶŹÉ«µÄSO2ĘųĢ壬Ņņ“Ė²½Öč2ÖŠĻČĻņĻ“µÓŗóµÄ¹ĢĢåµĪČė2µĪ»ņÉŁĮæĘ·ŗģČÜŅŗ£¬ŌŁµĪČė¹żĮæ2mol/LŃĪĖį£¬Õńµ“£¬Čō¹ĢĢåĶźČ«Čܽā£¬Ę·ŗģĶŹÉ«»ņÓŠĘųÅŻ£¬Ōņ×°ÖƱūµÄ²śĪļÖŠŗ¬ÓŠNa2SO3£»ÓÉÓŚæÉČÜŠŌµÄNaHSO3Ņ×ÓėBa(OH)2·“Ӧɜ³ÉBaSO3³Įµķ£¬»ņÕßNaHSO3Ņ×ÓėŃĪĖį·“Ó¦·Å³öÄÜŹ¹Ę·ŗģČÜŅŗĶŹÉ«µÄSO2ĘųĢ壬¹Ź²½Öč3ÖŠæÉÓĆŹŌ¹ÜČ”ŹŹĮæĀĖŅŗA£¬ĻņĘäÖŠ¼ÓČė¹żĮælmol/LĒāŃõ»Æ±µČÜŅŗ£Ø»ņµĪČė2µĪĘ·ŗģ£¬ŌŁµĪČė¹żĮæ2mol/LŃĪĖį£©£¬Õńµ“£¬Čō³öĻÖ»ė×Ē£Ø»ņŗģÉ«ĶŹČ„£©£¬Ōņ×°ÖƱūµÄ²śĪļÖŠ“ęŌŚNaHSO3£»·ńŌņ²»“ęŌŚNaHSO3”£
CuSO4+SO2”ü+2H2O£¬SO2²»½öŅ×ČÜÓŚĖ®£¬¶ųĒŅŅ×ČÜӌװÖƱūÖŠµÄNaOHČÜŅŗ£¬Ņņ“Ė±ūÖŠČÜŅŗŅ×µ¹Īü½ųČė¼××°ÖĆÄŚµÄÅØĮņĖįÖŠ£¬Ņ×Ōģ³É°²Č«ŹĀ¹Ź£¬ĖłŅŌ×°ÖĆŅŅµÄ×÷ÓĆ¾ĶŹĒ·ĄÖ¹±ūÖŠŅŗĢåµ¹ĪüČė×°ÖĆ¼×ÖŠ£Ø»ņĘš»ŗ³åĘæ»ņ°²Č«ĘæµÄ×÷ÓĆ£©£»£Ø2£©¶”×°ÖĆÖŠKMnO4×÷ĒæŃõ»Æ¼Į£¬SO2×÷»¹Ō¼Į£¬ĆĢŌŖĖŲÓÉ+7¼Ū½µĪŖ+2¼Ū£¬ĮņŌŖĖŲÓÉ+4¼ŪÉżĪŖ+6¼Ū£¬øł¾Ż»ÆŗĻ¼ŪÉż½µ×ÜŹżĻąµČ”¢Ō×ÓøöŹżŹŲŗćæÉÖŖ£¬2KMnO4+5SO2+2H2O=2MnSO4+K2SO4+2H2SO4£¬»ņÕß5SO2£«2MnO4££«2H2O=5SO42££«2Mn2+£«4H+£»£Ø3£©ĀČĖ®ŹĒĒæŃõ»Æ¼Į£¬æÉŅŌ½«SO2Ńõ»ÆĪŖSO42££¬¼“SO2+Cl2+2H2O=H2SO4+2HCl£¬ŌņSO2ŹĒ»¹Ō¼Į£¬ĻŌ»¹ŌŠŌ£»£Ø4£©ČōSO2²»×ć£¬±ūÖŠ·“Ó¦ĪŖSO2+2NaOH=Na2SO3+H2O£»ČōSO2¹żĮ棬±ūÖŠĻČ·¢ÉśµÄ·“Ó¦ĪŖSO2+2NaOH=Na2SO3+H2O£¬ŗó·¢ÉśµÄ·“Ó¦ĪŖNa2SO3+H2O+SO2=2NaHSO3£¬ÓÉ“ĖĶʶĻ±ūÖŠSO2¹żĮ棬ŌņµĪČėĘ·ŗģČÜŅŗ»įĶŹÉ«£¬ŅņĪŖ¶žŃõ»ÆĮņ¾ßÓŠĘư׊Ō£»£Ø5£©Na2SO3ÓėBaCl2Ņ×·“Ӧɜ³ÉBaSO3³Įµķ£¬¶ųNaHSO3ÓėBaCl2²»ÄÜ·“Ó¦£»BaSO3Ņ×ČÜÓŚŃĪĖį£¬²¢·Å³öÄÜŹ¹Ę·ŗģČÜŅŗĶŹÉ«µÄSO2ĘųĢ壬Ņņ“Ė²½Öč2ÖŠĻČĻņĻ“µÓŗóµÄ¹ĢĢåµĪČė2µĪ»ņÉŁĮæĘ·ŗģČÜŅŗ£¬ŌŁµĪČė¹żĮæ2mol/LŃĪĖį£¬Õńµ“£¬Čō¹ĢĢåĶźČ«Čܽā£¬Ę·ŗģĶŹÉ«»ņÓŠĘųÅŻ£¬Ōņ×°ÖƱūµÄ²śĪļÖŠŗ¬ÓŠNa2SO3£»ÓÉÓŚæÉČÜŠŌµÄNaHSO3Ņ×ÓėBa(OH)2·“Ӧɜ³ÉBaSO3³Įµķ£¬»ņÕßNaHSO3Ņ×ÓėŃĪĖį·“Ó¦·Å³öÄÜŹ¹Ę·ŗģČÜŅŗĶŹÉ«µÄSO2ĘųĢ壬¹Ź²½Öč3ÖŠæÉÓĆŹŌ¹ÜČ”ŹŹĮæĀĖŅŗA£¬ĻņĘäÖŠ¼ÓČė¹żĮælmol/LĒāŃõ»Æ±µČÜŅŗ£Ø»ņµĪČė2µĪĘ·ŗģ£¬ŌŁµĪČė¹żĮæ2mol/LŃĪĖį£©£¬Õńµ“£¬Čō³öĻÖ»ė×Ē£Ø»ņŗģÉ«ĶŹČ„£©£¬Ōņ×°ÖƱūµÄ²śĪļÖŠ“ęŌŚNaHSO3£»·ńŌņ²»“ęŌŚNaHSO3”£


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
| µĪ¶Ø“ĪŹż | “ż²āČÜŅŗ Ģå»ż/mL | ²ŻĖįČÜŅŗĢå»ż/mL | |
| µĪ¶ØĒ°æĢ¶Č | µĪ¶ØŗóæĢ¶Č | ||
| 1 | 25.00 | 1.50 | 23.70 |
| 2 | 25.00 | 1.02 | 26.03 |
| 3 | 25.00 | 0.00 | 24.99 |
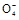 +2H2O+5SO2
+2H2O+5SO2 2Mn2++5S
2Mn2++5S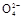 +4H+
+4H+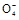 +6H++5H2C2O4
+6H++5H2C2O4 2Mn2++10CO2ӟ+8H2O
2Mn2++10CO2”ü+8H2O| ±ąŗÅ | ĪĀ¶Č/”ę | Ėį»ÆµÄH2C2O4 ČÜŅŗ/mL | KMnO4 ČÜŅŗ/mL | ČÜŅŗĶŹ É«Ź±¼ä/s |
| 1 | 25 | 5.0 | 2.0 | 40 |
| 2 | 25 | 5.0(Įķ¼ÓÉŁĮææÉČÜ ÓŚĖ®µÄMnSO4·ŪÄ©) | 2.0 | 4 |
| 3 | 60 | 5.0 | 2.0 | 25 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ŹµŃé¢ńŹŌ¹ÜÖŠŗģÉ«ČÜŅŗÖš½„±ä³ÉĪŽÉ«ČÜŅŗ |
| B£®ŹµŃé¢ņŹŌ¹ÜÖŠ³öĻÖµ»ĘÉ«»ė×Ē |
| C£®ŹµŃé¢óĢś°ōÉĻÓŠĪŽÉ«ĘųÅŻ²śÉś |
| D£®ŹµŃé¢ōÖŠĖįŠŌKMnO4ČÜŅŗÖŠ³öĻÖĘųÅŻĒŅŃÕÉ«Öš½„ĶŹČ„ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĪŹ“šĢā
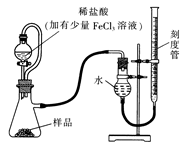
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®³żČ„NaClČÜŅŗÖŠµÄCl2£¬æɼÓČė¾Ę¾«ŗó·ÖŅŗ |
| B£®µĪ¶Ø¹ÜÓĆÕōĮóĖ®Ļ“¾»ŗó£¬Ö±½Ó×°Čė±ź×¼ÅØ¶ČµÄČÜŅŗµĪ¶Ø |
| C£®ÓĆÅÅĖ®·ØŹÕ¼ÆĻ”HNO3ŗĶCu·“Ó¦²śÉśµÄNOĘųĢå |
| D£®ÓĆKSCNČÜŅŗŗĶĀČĖ®¼ų±šFeCl3ČÜŅŗÖŠŹĒ·ńŗ¬ÓŠFeCl2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
 CuO + SO3ӟ 2SO3
CuO + SO3ӟ 2SO3  2SO2ӟ+ O2ӟ
2SO2ӟ+ O2ӟ
 2SO2(g) + O2(g)µÄĘ½ŗā³£Źż±ķ“ļŹ½ĪŖK= ”£
2SO2(g) + O2(g)µÄĘ½ŗā³£Źż±ķ“ļŹ½ĪŖK= ”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ĪŖ¼ų±šKCl”¢AlCl3ŗĶMgCl2ČÜŅŗ£¬·Ö±šĻņČżÖÖČÜŅŗÖŠµĪ¼ÓNaOHČÜŅŗÖĮ¹żĮæ |
| B£®Óū³żČ„µ°°×ÖŹČÜŅŗÖŠµÄNaCl¶ųÓÖ²»øıäĘäŠŌÖŹ£¬æɼÓČėŹŹĮæBaCl2ČÜŅŗŗó¹żĀĖ |
| C£®²āĀČĖ®µÄpH£¬æÉÓĆ²£Į§°ōÕŗČ”ĀČĖ®µćŌŚpHŹŌÖ½ÉĻ£¬“żĘä±äÉ«ŗóŗĶ±ź×¼±ČÉ«æØ±Č½Ļ |
| D£®ĪŖĮĖÖ¤Ć÷½¹ĀÆĘųÖŠŗ¬ÓŠĒāĘų£¬æɽ«½¹ĀÆĘųĶعż×ĘČȵÄŃõ»ÆĶ·ŪÄ©£¬æ“ŗŚÉ«·ŪÄ©ŹĒ·ń±äŗģÉ« |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®1mol”¤L£1”” | B£®0.045mol”¤L£1 | C£®0.029mol”¤L£1 | D£®²»ÄÜČ·¶Ø |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com