
| n |
| V |
| 1 |
| 3 |
| n |
| V |
| n |
| V |
| n |
| V |
| n |
| V |
| n |
| V |
| n |
| V |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
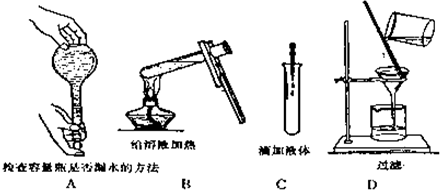
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��18mol/L��Ũ��������80mL 1.0mol/L��ϡ���ᣬ���õ�ʵ�������У�A.100mL��Ͳ��B��������ƽ��C����������D��50mL����ƿ��E��10mL��Ͳ��F����ͷ�ιܡ�G��50mL�ձ���H��100mL����ƿ
��18mol/L��Ũ��������80mL 1.0mol/L��ϡ���ᣬ���õ�ʵ�������У�A.100mL��Ͳ��B��������ƽ��C����������D��50mL����ƿ��E��10mL��Ͳ��F����ͷ�ιܡ�G��50mL�ձ���H��100mL����ƿ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ����Է�������Ϊ204.0���dz��õĻ����ʣ�ijѧ�����ڱ���������زⶨδ֪NaOH��Һ��Ũ�ȣ��ڱ�ʵ���дﵽ�ζ��յ�ʱ����Һ��pHԼΪ9.1��
����Է�������Ϊ204.0���dz��õĻ����ʣ�ijѧ�����ڱ���������زⶨδ֪NaOH��Һ��Ũ�ȣ��ڱ�ʵ���дﵽ�ζ��յ�ʱ����Һ��pHԼΪ9.1��| ʵ���� | NaOH��Һ�������mL�� |
| 1 | 22.52 |
| 2 | 22.49 |
| 3 | 22.50 |
| ||
(
|
| ||
(
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼΪ������Һ�õ�������ˮ����ѧʽΪNaCl��ע��Һ��ǩ�ϵIJ��փ��ݣ�����ע��Һ�����Ȼ��Ƶ�ˮ��Һ���Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
��ͼΪ������Һ�õ�������ˮ����ѧʽΪNaCl��ע��Һ��ǩ�ϵIJ��փ��ݣ�����ע��Һ�����Ȼ��Ƶ�ˮ��Һ���Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �¶ȣ��棩 | 10 | 20 | 30 | 50 | 70 | 80 | 100 |
| pH | 8.3 | 8.4 | 8.5 | 8.9 | 9.4 | 9.6 | 10.1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijѧ����ͨ���ⶨ��п��������ϡ�����Ӧ����ϡ���ᷴӦ������������������ⶨ��п��п�Ĵ��ȡ����ô�п��ϡ���ᡢ�Թܡ����ӡ�������Ƥ���������ܡ�������ƽ����Ͳ��ˮ�ۡ�����̨�������У���������ҩƷ����ʵ�顣ʵ��װ����ͼ��ʾ���̶�װ��δ��������
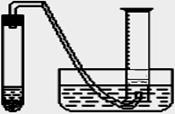
��1���÷�Ӧ�����ӷ���ʽΪ ��
��2��ijѧ������11.9 mol/L Ũ���������ˮ����500 mL���ʵ���Ũ��Ϊ0��400 mol��L��ϡ���ᡣ��ѧ����Ҫ����Ͳ��ȡ mL����Ũ����������ơ�
��3��ʵ��ʱ����ⶨ��п�����������������֮�⣬�Ƿ���Ҫ����������������Ҫ����ָ��____________________�����˿ղ��
��4���ڼ���ҩƷ֮ǰ����װ�������ԣ���μ���װ�õ������ԣ�
��
��5�������Dz����ռ������������������ļ������裺
�ٵ�����Ͳ����Һ��߶�ʹ֮��ͬ��
��ʹ�Թܺ���Ͳ�ڵ����嶼��ȴ�����£�
�۶�ȡ��Ͳ������������
��������������ȷ˳���ǣ�________________________������д������ţ���
��6�����ʵ���в�ô�п����Ϊa g���õ������������b L��������ɱ�״������ˮ������Ӱ����Բ��ƣ���п��п�����������ļ���ʽΪ���ú�a��b�Ĵ���ʽ��ʾ�����ػ���___________________��
��7���������һ�ָ���ݵ�ʵ�鷽����________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com