
����Ŀ����֪X��Y��Z����Ԫ�ص�ԭ������֮�͵���42��XԪ��ԭ�ӵ�4p�������3��δ�ɶԵ��ӣ�YԪ��ԭ�ӵ������2p�������2��δ�ɶԵ��ӡ�X��Y���γɻ�����X2Y3��ZԪ�ؿ����γɸ�һ�����ӡ���ش��������⣺
(1)XԪ��ԭ�ӻ�̬ʱ�ĵ����Ų�ʽΪ_______________��
(2)YԪ��ԭ�ӵļ۲���ӵĹ����ʾʽΪ_____________��
(3)X��Z���γɻ�����XZ3���û�����Ŀռ乹��Ϊ________��
(4)��֪������X2Y3��ϡ������Һ�пɱ�����п��ԭΪXZ3�����ﻹ��ZnSO4��H2O���÷�Ӧ�Ļ�ѧ����ʽ��___________��
(5)�Ƚ�X���⻯����ͬ��ڶ�����������Ԫ�����γɵ��⻯��е�ߵ�______��
���𰸡�1s22s22p63s23p63d104s24p3 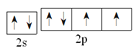 ������ As2O3+6Zn+6H2SO4=2AsH3��+6ZnSO4+3H2O NH3>AsH3>PH3
������ As2O3+6Zn+6H2SO4=2AsH3��+6ZnSO4+3H2O NH3>AsH3>PH3
��������
XԪ��ԭ�ӵ�4p�������3��δ�ɶԵ��ӣ�XԪ��ԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d104s24p3�����ڵ������ڵ�VA�壬��XΪAsԪ�أ�YԪ��ԭ�ӵ������2p�������2��δ�ɶԵ��ӣ�Y��2p�������2�����ӻ�4�����ӣ�����YΪ̼Ԫ�ػ���Ԫ�أ�X��Y���γɻ�����X2Y3����YΪOԪ�أ�X��Y��Z����Ԫ�ص�ԭ������֮�͵���42����Z��������Ϊ42-8-33=1����ZΪHԪ�أ���ԭ�ӿ����γɸ�һ�����ӣ��������⣬Ȼ����һ�������
��������������֪��X��AsԪ�أ�Y��OԪ�أ�Z��HԪ�ء�
(1)X ��AsԪ�أ�XԪ��ԭ�ӻ�̬ʱ�ĵ����Ų�ʽΪ��1s22s22p63s23p63d104s24p3��
(2)Y��OԪ�أ���������Ų�ʽΪ1s22s22p4���۲�����Ų�ʽ��2s22p4����������ʾʽΪ�� ��
��
(3)X��As��Z��H��As��H����Ԫ���Թ��ۼ��γ�AsH3������As��NΪͬ����Ԫ�أ�AsH3�ռ�ṹ��NH3���ƣ��������ͣ�
(4)X��As��Y��O�������γ�As2O3��Zn��H2SO4��Ӧ����AsH3��ZnSO4��H2O������ԭ���غ㡢�����غ㣬�ɵ÷�Ӧ����ʽΪ��As2O3+6Zn+6H2SO4=2AsH3��+6ZnSO4+3H2O��
(5)As�ǵ������ڵ�VA��Ԫ�أ��������е�VA��Ԫ�ػ���N��P�������γɵ������ֱ���AsH3��NH3��PH3�����������Ϊ���Ӿ��壬����֮��ͨ�����Ӽ���������ϣ����ʵ���Է�������Խ���Ӽ�������Խ�����ʵ��۷е��Խ�ߡ�������NH3�ķ��Ӽ��������������˷���֮�����������ʹNH3�ķе���ߣ����������ʷе��ɸߵ��͵�˳���ǣ�NH3>AsH3>PH3��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧС���Ի������Ʊ�����ϩ����ͼ����
��֪�� +H2O
+H2O



��1���Ʊ���Ʒ
��12.5mL�����������Թ�A�У��ټ���1mLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
��A�����Ƭ��������_______��
������B��������______��
��2���Ʊ���Ʒ
������ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ��____�㣨���ϻ��£�����Һ����___������ĸ��ϴ�ӣ�
a��KMnO4��Һ b��ϡH2SO4 c��Na2CO3��Һ
���ٽ�����ϩ����ͼװ��������ȴˮ��___�ڽ��루����ĸ��������ʱҪ������ʯ�ң�Ŀ����____��
���ռ���Ʒʱ�����Ƶ��¶�Ӧ��______���ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ����_____������ĸ����
a������ʱ��70����ʼ�ռ���Ʒ
b��������ʵ����������
c���Ʊ���Ʒʱ���������Ʒһ������
��3���������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ�������������_____������ĸ����
a�������Ը��������Һ b���ý����� c���ⶨ�е�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������仯�����ڹ�ũҵ���������������ж�������Ҫ��Ӧ�á�
(1)NaClO�н�ǿ�������ԣ������dz��õ���������Ư������д����ҵ����������NaOH��Һ����������NaClO�����ӷ���ʽ_________________________��
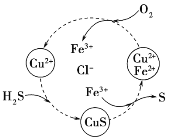
(2)��H2S�Ϳ����Ļ������ͨ��FeCl3��FeCl2��CuCl2�Ļ����Һ�з�Ӧ���Ի���S��������ת����ͼ��ʾ����ͼʾ���ܷ�ӦΪ_________________________��
(3)����ˮ�е�NO3-�����ཡ������Σ����Ϊ�˽�������ˮ��NO3-��Ũ�ȣ�ij�о���Ա����ڼ�����������Al�ۻ�ԭNO3-��������N2�������ķ�Ӧ�ɱ�ʾ���£�����ɷ���ʽ����ƽ��
___Al+___NO3-+___ =___AlO2-+___N2��+___H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼ��ա�
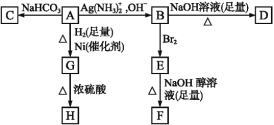
��֪H�ǻ�״������C4H6O2,F��̼ԭ�Ӷ���һ��ֱ���ϡ�
��1��������A���еĹ������� ��
��2��B��������������Br2��Ӧ�õ�E,E���������������ƴ���Һ������ת���F,��Eת���Fʱ�������ַ�Ӧ,�䷴Ӧ���ͷֱ��� ��
��3��D�Ľṹ��ʽ�� ��
��4��1 mol A��2 mol H2��Ӧ����1 mol G,�䷴Ӧ����ʽ�� ��
��5����A������ͬ�����ŵ�A��ͬ���칹��Ľṹ��ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������YX2��ZX2�У�X��Y��Z�ĺ˵����С��18��Xԭ�������ܲ��p�ܼ�����һ�����������2�����ӣ�Yԭ�ӵ��������p�ܼ��ĵ���������ǰһ�ܲ������������X��Y������ͬ�ĵ��Ӳ㣻Z��X�����ڱ���λ��ͬһ���塣�ش��������⣺
(1)X�ĵ����Ų�ʽΪ_______��Y�Ĺ����ʾʽΪ_______��
(2)ZX2�ķ���ʽ��_______��YX2����ʽ��_________��
(3)Y��Z�γɵĻ�����ķ���ʽ��_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��úȼ���ŷŵ���������SO2��NOx������NaClO2��Һ��Ϊ���ռ���ͬʱ�����������������������������գ�
��1���������������������漰���Ļ�ѧ�������Ԫ���У����ڵ�������Ԫ�ص���___��д��N�ĺ�������Ų�ʽ___��
��2����֪SO2���ӵĿռ乹��Ϊ�����Σ���SO2Ϊ___��ѡ���������������Ǽ����������ӡ�
��3��������SO2��NOx������ͨ��ʢ��NaClO2��Һ�ķ�Ӧ���У���Ӧһ��ʱ�ʺ����Һ������Ũ�ȵ��й��������£��������Ӻ��Բ��ƣ���
���� | Na+ | SO42- | NO3- | OH- | Cl- |
Ũ��/��mol��L-1�� | 5.5��10-3 | 8.5��10-4 | y | 2.0��10-4 | 3.4��10-3 |
�ٷ�Ӧ����ҺpH___7������y=___mol��L-1��
��д��NaClO2��Һ����SO2�����ӷ���ʽ___��
��4�������е�SO2���ɲ��ð��������ȥ���䷴Ӧԭ��������ͼ��ʾ��
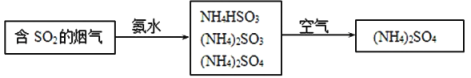
��д��SO2����ˮ��Ӧ����NH4HSO3�Ļ�ѧ����ʽ___��
��(NH4)2SO4��Һ��Ũ������������___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ѽ��仯������о���Ӧ��Խ��Խ�ܵ����ǵ����ӡ�
(1)Ti(BH4)2��һ�ֹ���Ԫ�����⻯�ﴢ����ϣ�����TiCl4��LiBH4��Ӧ�Ƶá�
���ڻ�̬Ti2���У�����ռ�ݵ�����ܲ����Ϊ_____�����ܲ���е�ԭ�ӹ����Ϊ____
��LiBH4��Li����BH4-���ɣ�BH4-������ṹ��____�����ݻ�����LiBH4�жϣ�Li��B��H�ĵ縺���ɴ�С��˳��Ϊ_____��
��TiCl4�ڳ���������ɫҺ�壬��TiCl4����_______(����ԭ����������������������)���塣
(2)�߷������������ް��ǽ���������̫��������䣬���������������Լ�ǿ�Ļ������ӣ����ֻ������ӿ��Էֽ������е�һЩ�к�����(�籽����ȩ����ͪ��)��
�ٱ�ͪ(![]() )�����к���������������Ŀ֮��Ϊ______��
)�����к���������������Ŀ֮��Ϊ______��
�ڼ�ȩ(![]() )������Cԭ�ӹ���ӻ�����Ϊ_______��
)������Cԭ�ӹ���ӻ�����Ϊ_______��
��ȩ������ˮ��ԭ���ǣ�a.��ȩ��ˮ���Ǽ��Է��ӣ�b._______��
(3)ij�ֵ����Ѿ���ľ�����ͼ��ʾ���þ�������Nԭ�Ӿ�������������Nԭ����__����Tiԭ�ӵ���λ��Ϊ______���þ�����N��Tiԭ��֮����������Ϊa nm����õ����Ѿ�����ܶ�Ϊ_______g��cm��3(NAΪ�����ӵ�������ֵ��ֻ�м���ʽ)��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�������µ��ܱ������У�4NH3(g)+5O2(g)![]() 4NO(g)+6H2O(g) ��H=-905.9kJ��mol-1������������ȷ����
4NO(g)+6H2O(g) ��H=-905.9kJ��mol-1������������ȷ����
A.4molNH3��5molO2��Ӧ���ﵽƽ��ʱ�ų�����Ϊ905.9kJ
B.ƽ��ʱ��5v��(O2)=4v��(NO)
C.ƽ���ѹǿ���������ƽ��Ħ����������
D.ƽ��������¶ȣ����������NO��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ijЩ���������˲�ͬ�̶ȵ�������������У����С���ͬѧ��������˵����о�,
��.����С���о�����,������Ҫ��Ⱦ��Ϊ�����������PM2.5������Ҫ��ԴΪȼú��������β���ȡ�
(1)25��ʱ��PM2.5����������ˮ�����Ƴɴ�����Һ����ø���������ˮ���������ӵĻ�ѧ��ּ���ƽ��Ũ�����±���

���ݱ��������ж�������pH=________��
��.���������������������һ���о�
һ�������������������о�
(1)һ�������£���2molNO��2molO2���ں����ܱ������з�����Ӧ��
2NO(g)+O2(g) ![]() 2NO2(g)������״̬��˵���÷�Ӧ�ﵽ��ѧƽ�����_______������ĸ��ţ���
2NO2(g)������״̬��˵���÷�Ӧ�ﵽ��ѧƽ�����_______������ĸ��ţ���
A�����������ܶȱ��ֲ��� B��NO��ת���ʱ��ֲ���
C��NO��O2�����ʵ���֮�ȱ��ֲ��� D��O2���������ʺ�NO2�������������
(2)����̿�ɴ���������Ⱦ��NO����Ӧԭ��Ϊ��C(s)+2NO(g)![]() N2(g)+CO2(g)��T��ʱ����2L�ܱ������м���0.100mol NO��2.030mol����̿�������ʣ���ƽ��ʱ����̿���ʵ�����2.000mol������¶��µ�ƽ�ⳣ��Ϊ_________����ƽ��ʱ,�����������г���0.04molNO,0.03molN2��0.03molCO2����ƽ��________�ƶ���������������������������������
N2(g)+CO2(g)��T��ʱ����2L�ܱ������м���0.100mol NO��2.030mol����̿�������ʣ���ƽ��ʱ����̿���ʵ�����2.000mol������¶��µ�ƽ�ⳣ��Ϊ_________����ƽ��ʱ,�����������г���0.04molNO,0.03molN2��0.03molCO2����ƽ��________�ƶ���������������������������������
III.һ����̼��������̼���о�
(1)CO��CO2��һ�������¿��������ϳɼ״�����ҵ�ϳ���CO��H2�Ʊ�CH3OH�ķ�ӦΪ��CO(g)+2H2(g)![]() CH3OH(g)�������Ϊ1L�ĺ����ܱ������У�����2molCO��4molH2��һ�������·���������Ӧ�����CO(g)��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ���ӷ�Ӧ��ʼ��5min����H2��ʾ��ƽ����Ӧ����v(H2)=_____��
CH3OH(g)�������Ϊ1L�ĺ����ܱ������У�����2molCO��4molH2��һ�������·���������Ӧ�����CO(g)��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ���ӷ�Ӧ��ʼ��5min����H2��ʾ��ƽ����Ӧ����v(H2)=_____��

(2)̼��ˮ������Ӧ��ȡH2����ط�Ӧ���£�
��C(s)+H2O(g)=CO(g)+H2(g) �� H=+131.0kJ/mol
��CO(g)+H2O(g)=CO2(g)+H2(g) �� H= - 43kJ/mol
��CaO(s)+CO2(g)=CaCO3(S) �� H= - 178.3kJ/mol
�ټ��㷴Ӧ����C(s)+2H2O(g)+CaO(s)![]() CaCO3(s)+2H2(g)����H=______kJ/mol��
CaCO3(s)+2H2(g)����H=______kJ/mol��
�������������о�
��SO2�ɱ�Na2FeO4������ȥ����Ⱦ����ҵ����Fe��ʯī���缫���ŨNaOH��Һ�Ʊ�Na2FeO4��д�������������������ĵ缫��Ӧ________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com