


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��18�֣�
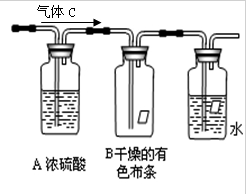
����ͭ��һ��Ӧ�ü���㷺�Ļ���ԭ�ϣ�ij������ȤС�齫����Ũ����ֶ�μӵ�ͭ����ϡ ����Ļ�����У�����ʹ֮��Ӧ��ȫ��װ������29ͼI��ͼ����ʾ����ͨ���������ᾧ�õ�����ͭ���壬��ͬʱ�ⶨ����ͭ�����нᾧˮ�ĺ�����
��1���������ͼIװ�õ������� ��
��2��ͼI�з�Һ©����װ��Һ���� ��
��3��ͼ����ͼ��ĸĽ�װ�ã���ͼI��ȣ�ͼ��װ�õ������ŵ��� ��
�� �����ɲ���������
��4��������ȤС��ͬѧ�ڲⶨ����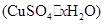 �����нᾧˮ�ĺ���ʱ������й��������±���
�����нᾧˮ�ĺ���ʱ������й��������±���
| ����ǰ���� | ���Ⱥ����� | |
| m1�������� | m2������+���壩 | m3������+��ˮCuSO4�� |
| 5.4g | 7.9g | 6.8g |
��Ϊ��ɲⶨ����29ͼ���л�ȱ�ٵ����������� ��
���ж���Ʒ�Ѻ��صķ����� ��
�۲ⶨ���õ���![]() �нᾧˮ������ʵ���У������������ٽ��� �Ρ�
�нᾧˮ������ʵ���У������������ٽ��� �Ρ�
�ܿ�����ȤС��IJⶨ���xֵ���� ��ȣ� ���ƫ�ߡ�����ƫ�͡��������䡱�������ܵ�ԭ���� ��������ĸ��ţ�
��ȣ� ���ƫ�ߡ�����ƫ�͡��������䡱�������ܵ�ԭ���� ��������ĸ��ţ�
a�������¶ȹ��� b����������Ŀ����ϴ�
c�����Ⱥ���ڿ�������ȴ d���������岿�ַ绯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣�����ͭ��һ��Ӧ�ü���㷺�Ļ���ԭ�ϣ�ij������ȤС�齫����Ũ����ֶ�μӵ�ͭ����ϡ����Ļ�����У�����ʹ֮��Ӧ��ȫ��װ����ͼI��ͼ����ʾ����ͨ���������ᾧ�õ�����ͭ���壬��ͬʱ�ⶨ����ͭ�����нᾧˮ�ĺ�����
��1���������ͼIװ�õ������� ��
��2��ͼI�з�Һ©����װ��Һ���� ��
��3��ͼ����ͼ��ĸĽ�װ�ã���ͼI��ȣ�ͼ��װ�õ������ŵ��� ��
�� �����ɲ���������
��4��������ȤС��ͬѧ�ڲⶨ������CuSO4����H2O�������нᾧˮ�ĺ���ʱ������й��������±���
| ����ǰ���� | ���Ⱥ����� | |
| m1�������� | m2������+���壩 | m3������+��ˮCuSO4�� |
| 5.4g | 7.9g | 6.8g |
��Ϊ��ɲⶨ��ͼ���л�ȱ�ٵ����������� ��
���ж���Ʒ�Ѻ��صķ����� ��
�۲ⶨ���õ�����CuSO4����H2O���нᾧˮ������ʵ���У������������ٽ��� �Ρ�
�ܿ�����ȤС��IJⶨ���xֵ����CuSO4��5H2O��ȣ� ���ƫ�ߡ�����ƫ�͡��������䡱�������ܵ�ԭ���� ��������ĸ��ţ�
a�������¶ȹ���
b����������Ŀ����ϴ�
c�����Ⱥ���ڿ�������ȴ
d���������岿�ַ绯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���������Ͽ���ѧ������ǰ��һ��ģ�⣨���ۣ���ѧ���� ���ͣ�ʵ����
��18�֣�
����ͭ��һ��Ӧ�ü���㷺�Ļ���ԭ�ϣ�ij������ȤС�齫����Ũ����ֶ�μӵ�ͭ����ϡ ����Ļ�����У�����ʹ֮��Ӧ��ȫ��װ������29ͼI��ͼ����ʾ����ͨ���������ᾧ�õ�����ͭ���壬��ͬʱ�ⶨ����ͭ�����нᾧˮ�ĺ�����
��1���������ͼIװ�õ������� ��
��2��ͼI�з�Һ©����װ��Һ���� ��
��3��ͼ����ͼ��ĸĽ�װ�ã���ͼI��ȣ�ͼ��װ�õ������ŵ��� ��
�� �����ɲ���������
��4��������ȤС��ͬѧ�ڲⶨ���� �����нᾧˮ�ĺ���ʱ������й��������±���
�����нᾧˮ�ĺ���ʱ������й��������±���
| ����ǰ���� | ���Ⱥ����� | |
| m1�������� | m2������+���壩 | m3������+��ˮCuSO4�� |
| 5.4g | 7.9g | 6.8g |
 �нᾧˮ������ʵ���У������������ٽ��� �Ρ�
�нᾧˮ������ʵ���У������������ٽ��� �Ρ�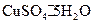 ���
��� �� ���ƫ�ߡ�����ƫ�͡��������䡱�������ܵ�ԭ���� ��������ĸ��ţ�
�� ���ƫ�ߡ�����ƫ�͡��������䡱�������ܵ�ԭ���� ��������ĸ��ţ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�������и�����ǰ��һ��ģ�⣨���ۣ���ѧ���� ���ͣ�ʵ����
��18�֣�
����ͭ��һ��Ӧ�ü���㷺�Ļ���ԭ�ϣ�ij������ȤС�齫����Ũ����ֶ�μӵ�ͭ����ϡ ����Ļ�����У�����ʹ֮��Ӧ��ȫ��װ������29ͼI��ͼ����ʾ����ͨ���������ᾧ�õ�����ͭ���壬��ͬʱ�ⶨ����ͭ�����нᾧˮ�ĺ�����

��1���������ͼIװ�õ������� ��
��2��ͼI�з�Һ©����װ��Һ���� ��
��3��ͼ����ͼ��ĸĽ�װ�ã���ͼI��ȣ�ͼ��װ�õ������ŵ��� ��
�� �����ɲ���������
��4��������ȤС��ͬѧ�ڲⶨ����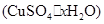 �����нᾧˮ�ĺ���ʱ������й��������±���
�����нᾧˮ�ĺ���ʱ������й��������±���
|
����ǰ���� |
���Ⱥ����� |
|
|
m1�������� |
m2������+���壩 |
m3������+��ˮCuSO4�� |
|
5.4g |
7.9g |
6.8g |
��Ϊ��ɲⶨ����29ͼ���л�ȱ�ٵ����������� ��
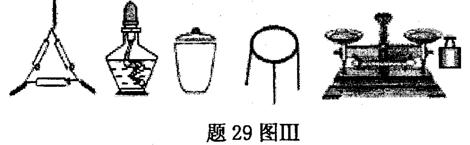
���ж���Ʒ�Ѻ��صķ����� ��
�۲ⶨ���õ���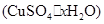 �нᾧˮ������ʵ���У������������ٽ��� �Ρ�
�нᾧˮ������ʵ���У������������ٽ��� �Ρ�
�ܿ�����ȤС��IJⶨ���xֵ����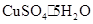 ��ȣ�
���ƫ�ߡ�����ƫ�͡��������䡱�������ܵ�ԭ����
��������ĸ��ţ�
��ȣ�
���ƫ�ߡ�����ƫ�͡��������䡱�������ܵ�ԭ����
��������ĸ��ţ�
a�������¶ȹ��� b����������Ŀ����ϴ�
c�����Ⱥ���ڿ�������ȴ d���������岿�ַ绯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�������и����^��ѧ���Ի�ѧ���������� ���ͣ�ʵ����
��12�֣�����ͭ��һ��Ӧ�ü���㷺�Ļ���ԭ�ϣ�ij������ȤС�齫����Ũ����ֶ�μӵ�ͭ����ϡ����Ļ�����У�����ʹ֮��Ӧ��ȫ��װ����ͼI��ͼ����ʾ����ͨ���������ᾧ�õ�����ͭ���壬��ͬʱ�ⶨ����ͭ�����нᾧˮ�ĺ�����

��1���������ͼIװ�õ������� ��
��2��ͼI�з�Һ©����װ��Һ���� ��
��3��ͼ����ͼ��ĸĽ�װ�ã���ͼI��ȣ�ͼ��װ�õ������ŵ��� ��
�� �����ɲ���������
��4��������ȤС��ͬѧ�ڲⶨ������CuSO4����H2O�������нᾧˮ�ĺ���ʱ������й��������±���
|
����ǰ���� |
���Ⱥ����� |
|
|
m1�������� |
m2������+���壩 |
m3������+��ˮCuSO4�� |
|
5.4g |
7.9g |
6.8g |
��Ϊ��ɲⶨ��ͼ���л�ȱ�ٵ����������� ��

���ж���Ʒ�Ѻ��صķ����� ��
�۲ⶨ���õ�����CuSO4����H2O���нᾧˮ������ʵ���У������������ٽ��� �Ρ�
�ܿ�����ȤС��IJⶨ���xֵ����CuSO4��5H2O��ȣ� ���ƫ�ߡ�����ƫ�͡��������䡱�������ܵ�ԭ���� ��������ĸ��ţ�
a�������¶ȹ���
b����������Ŀ����ϴ�
c�����Ⱥ���ڿ�������ȴ
d���������岿�ַ绯
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com