

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ɽ��ʡ�����и�����ѧ�������У�һģ����ѧ�Ծ� ���ͣ������
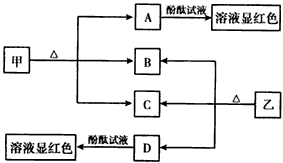
��7�֣���ͼ����������ѧ��ѧ�г��������ʣ��ס��Ҿ������ӻ���������������Ӹ�����Ϊ1��1�����Ƿ��ͷ۵���Ҫ�ɷ֣�����һ�ֳ��õĻ��ʡ�B��D���³�ѹ�������塣��ش��������⣺
��1����������____________________ ________��
________��
��2�� A��D���ʵ�ˮ��Һ�ֱ�����̪��Һ����Һ���Ժ� ɫ��˵����Һ�����ԣ���ԭ���Ƿ���ͬ�����ñ�Ҫ�����ּ��Խ��Ͳ�д�����ӷ���ʽ��
ɫ��˵����Һ�����ԣ���ԭ���Ƿ���ͬ�����ñ�Ҫ�����ּ��Խ��Ͳ�д�����ӷ���ʽ��
__________________________________________________________��
��3�����Ȼ�����Һ��ͨ������D�����ͺ���ͨ������������B���Ƶ����ʼף�д���÷�Ӧ�Ļ�ѧ����ʽ��__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�꺣��ʡ�����и�����ѧ�ڽ�ѧ������⻯ѧ�Ծ��������棩 ���ͣ������
��ͼ����������ѧ��ѧ�г��������ʣ��ס��Ҿ������ӻ���������������Ӹ�����Ϊ1��1���������ͷۣ�����һ�ֳ��õĻ��ʡ�B��D�����������塣��ش��������⣺

��1����������____________��
��2��D��ˮ��Һ�����̪��Һ����Һ�Ժ�ɫ�����ñ�Ҫ�����ּ��Խ��Ͳ�д����ص����ӷ���ʽ��___ _________��
��3������Һ�м����Ȼ�����Һ�����Թ۲쵽������Ϊ ��д����Ӧ�����ӷ���ʽ��____
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�콭��ʡ�����и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��ͼ����������ѧ��ѧ�г��������ʣ���A����ɫ��ӦΪ��ɫ�����³�ѹ��BΪ��ɫ��ζ���壬����ʹ�����ǵ�ľ����ȼ����ش��������⣺

��д��B�ĵ���ʽ ��
�ƹ�ҵ������D����ʯ������ij�����巴Ӧ��D������ ��
��д����A��Ļ�ѧ����ʽ ��
��B�������һ������������������CO�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�꺣��ʡ�����и�����ѧ�ڸ߿����в��ԣ�������ѧ�Ծ� ���ͣ������
��8�֣���ͼ����������ѧ��ѧ�г��������ʣ��ס��Ҿ������ӻ���������������Ӹ�����Ϊ1��1���������ͷۣ�����һ�ֳ��õĻ��ʡ�B��D�����������塣��ش��������⣺

��1����������________________��
��2��D��ˮ��Һ�����̪��Һ����Һ�Ժ�ɫ��˵��D��Һ��____�ԣ����ñ�Ҫ�����ּ��Խ��Ͳ�д����ص����ӷ���ʽ��____________��
��3������Һ�������KOH��Һ���ȣ���д����Ӧ�����ӷ���ʽ��_____________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com