
��1 mol H2(g)��2 mol I2(g)����ij2 L�ܱ������У���һ���¶��·�����Ӧ��
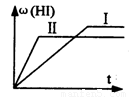
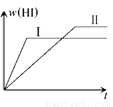
H2(g)��I2(g) 2HI(g)����H<0�����ﵽƽ�⡣HI���������w(HI)��ʱ��仯��ͼ(��)��ʾ�����ı䷴Ӧ������w(HI)�ı仯������ͼ(��)��ʾ����ı������������
2HI(g)����H<0�����ﵽƽ�⡣HI���������w(HI)��ʱ��仯��ͼ(��)��ʾ�����ı䷴Ӧ������w(HI)�ı仯������ͼ(��)��ʾ����ı������������

A�����º��������£������ʵ����� B�����������£���С��Ӧ�������
C�����������£������¶� D�����������£�����Ӧ�������
 ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1 mol H2(g)��2 mol I2(g)����ij2 L�ܱ������У���һ���¶��·�����Ӧ��
H2(g)��I2(g)2HI(g)����H<0�����ﵽƽ�⡣HI���������w(HI)��ʱ��仯��ͼ(��)��ʾ�����ı䷴Ӧ������w(HI)�ı仯������ͼ(��)��ʾ����ı������������
A�����º��������£������ʵ����� B�����������£���С��Ӧ�������
C�����������£������¶� D�����������£�����Ӧ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������������һ�и߶���ѧ�����п������ƻ�ѧ�Ծ����������� ���ͣ���ѡ��
��1 mol H2(g)��2 mol I2(g)����ij2 L�ܱ������У���һ���¶��·�����Ӧ��H2(g)+ I2(g) 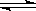 2HI(g)�� H��0,���ﵽƽ��,HI�����������(HI)��ʱ��仯��ͼ������ʾ�����ı䷴Ӧ������ ��(HI)�ı仯������ͼ������ʾ����ı������������
2HI(g)�� H��0,���ﵽƽ��,HI�����������(HI)��ʱ��仯��ͼ������ʾ�����ı䷴Ӧ������ ��(HI)�ı仯������ͼ������ʾ����ı������������ 
| A�����º��������£������ʵ����� |
| B�����������£���С��Ӧ������� |
| C�����������������¶� |
| D�����������£�����Ӧ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ��ˮ�и���ģ�⣨5�£����Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��1 mol H2��g����2 mol I2(g)����ij2L�ܱ������У���һ���¶��·������·�Ӧ�����ﵽƽ�⣺H2(g)+I2(g)
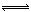 2HI��g������H��0��HI����������أ�HI����ʱ��仯��ͼ������ʾ�����ı䷴Ӧ�������أ�HI���ı仯������ͼ������ʾ����ı������������( )
2HI��g������H��0��HI����������أ�HI����ʱ��仯��ͼ������ʾ�����ı䷴Ӧ�������أ�HI���ı仯������ͼ������ʾ����ı������������( )
A.���������£������ʵ�����
B.���������£���С��Ӧ�������
C.���������������¶�
D.���������£�����Ӧ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012���㽭�������ո�������߶���ѧ������������ѧ�Ծ��������棩 ���ͣ�ѡ����
��1 mol H2(g)��2 mol I2(g)����ij2 L�ܱ������У���һ���¶��·�����Ӧ��
H2(g)��I2(g) 2HI(g)����H<0�����ﵽƽ�⡣HI���������w(HI)��ʱ��仯��ͼ(��)��ʾ�����ı䷴Ӧ������w(HI)�ı仯������ͼ(��)��ʾ����ı�����������ǣ�
��
2HI(g)����H<0�����ﵽƽ�⡣HI���������w(HI)��ʱ��仯��ͼ(��)��ʾ�����ı䷴Ӧ������w(HI)�ı仯������ͼ(��)��ʾ����ı�����������ǣ�
��
A�����º��������£������ʵ�����B�����������£���С��Ӧ�������
C�����������£������¶� D�����������£�����Ӧ�������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com