
ʵ����������MnO2��Ũ���ᷴӦ��ȡ��������װ����ͼ1��ʾ��

ͼ1 ͼ2
��1��ͼ1������a�������ǣ� ������b�������ǣ� ��b�м������Ƭ�������ǣ� ��
��2����д������b�з����ķ�Ӧ�����ӷ���ʽ��
��3�����װ�õ�������֮��IJ��������ǣ� �� �� ��������ţ�
A������ƿ�м���MnO2��ĩ
B������
C������ƿ�м���Ũ����
��4���÷�Ӧ����Ϊ����Ũ���½���ֹͣ��Ϊ�˲ⶨ��Ӧ����Һ�������Ũ�ȣ�ij̽��С���������ʵ�鷽����
�ټ�ͬѧ�ķ���Ϊ��������AgNO3��Һ��Ӧ���������ɳ�����������
����ͬѧ�ķ���Ϊ����������п��Ӧ��������������������ʵ��װ����ͼ2��ʾ���г�װ������ȥ����ʹY�ι��еIJ�����Һ��п����Ӧ����ȷ������ ����п��ת�Ƶ�������Һ�С�������Һת�Ƶ�п���С���������ȷ��ȡ�����ܶ���ʱ������Ҫƽ�ӣ�Ҫע��ʹ©��Һ������������Һ����ƽ���������ע�⣺ ��
���ַ�������Ϊ ������ң�ͬѧ�ķ������С�
��1����Һ©���� ������ƿ�� ��ֹҺ�屩��
��2��MnO2+4H++2Cl- Mn2++2H2O+Cl2��
Mn2++2H2O+Cl2��
��3��A��C��B
��4��п��ת����������Һ�У� �ָ������£� ��
�������������ͼ1װ���з�Һ©����������ƿ��װ���ɣ��������Ƭ��ֹ���У����������Ժ��ȼ�����壬�ټ�Һ�壬�����ȣ����¶��ϣ�MnO2+4H++2Cl- Mn2++2H2O+Cl2����������ֻ�ԭ�Ժ����ԣ���ͬѧ������������ӵ����������ij���ʣ������ᣬ���б������Ե����ᣬҲ�����Ȼ��̵������ӡ���ͬѧ��п��ת�������У���֤������ȫ��Ӧ��ȷ�ⶨ��������������ܶ���ʱע�⣺1���ظ������£�2������Һ����ƽ��3�������밼Һ�����С�
Mn2++2H2O+Cl2����������ֻ�ԭ�Ժ����ԣ���ͬѧ������������ӵ����������ij���ʣ������ᣬ���б������Ե����ᣬҲ�����Ȼ��̵������ӡ���ͬѧ��п��ת�������У���֤������ȫ��Ӧ��ȷ�ⶨ��������������ܶ���ʱע�⣺1���ظ������£�2������Һ����ƽ��3�������밼Һ�����С�
���㣺����ʵ����������������ʵ��װ�úͲ���


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧ��ȤС������ʵ����̽�����������ʼ�ģ�ҵ��ȡƯ�ۣ����������װ�ý���ʵ�飨ʵ������ȡ�����ķ�ӦΪMnO2 + 4 HCI(Ũ�� MnCl2 + C12��+ 2 H2O����
MnCl2 + C12��+ 2 H2O����
�밴Ҫ��ش��������⣺
��1�����۵⻯����Һ�й۲쵽��������_________����Ӧ�����ӷ���ʽ____________��
��2�����������ɫ��������ɫ��ʪ�����ɫ������ɫ�������ʢ��________�������ƣ���
��3��C12��ʯ���鷴Ӧ��ȡƯ�۵Ļ�ѧ����ʽΪ___________________��
��4������ȤС����8.7g MnO2��������Ũ�����Ʊ��������������������Ƶñ�״���µ�Cl2______________L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
Ϊ��̽��AgNO3�������Ժ����ȶ��ԣ�ij��ѧ��ȤС�����������ʵ�顣
��.AgNO3��������
����������˿����AgNO3��Һ�У�һ��ʱ�����˿ȡ����Ϊ������Һ��Fe�������������Һ�е�Ag����������������ʵ�顣��ѡ�õ��Լ���KSCN��Һ����ˮ��
��1��������±���
| ���� | ���� | ���� |
| ȡ��������Ag�������Һ���Թ��У�����KSCN��Һ���� | ____ | ����Fe3�� |
| ʵ���� | ���� | ���� |
| a | ����������ˮ���� | ��ɫ���岻�ܽ� |
| b | ��������ϡ���ᣬ�� | ��ɫ�����ܽ⣬����������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
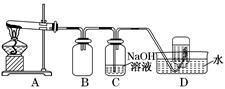
ij��ѧ����С����ʵ�����������ͼ��ʾ��ʵ��װ�ã����С����Ĵ�������ʵ�顣
��1��A�������巢��װ�ã�A�����õ��Լ�ֻ�ܴ�����������ѡȡ��
������泥���̼��泥���̼����泥����Ȼ�泥�����ʯ�ң����������ơ�
��A����ȡ����ʱֻ����һ��ҩƷ�����ҩƷ������_________����ѡ���ţ�����ֻ��һ��ҩƷ��ȡ����ʱ��ͼ�пհ״���������ӦΪ_________ ��ѡ������������ţ��̶�װ��ʡ�ԣ���
��2����װ�ò�����������Ȼ����һ����ȱ�ݣ��ԴӰ�ȫ�뻷���ĽǶ������ǣ��Ը�װ�ý��иĽ���
��_______________________________________________________________;
��_______________________________________________________________��
��3�����ոĽ����װ�ý���ʵ�飬������������⣺
��װ��B��������__________________________________________________��
��д��C�з�����Ӧ�Ļ�ѧ����ʽ�� ________________________________��
����A��B���Լ���������װ��D�п��Թ۲쵽��ʵ��������_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij������ȤС���������л�ѧ�Լ���ʵ��������ȡCl2����֤Cl2��ijЩ��ѧ���ʡ�ʵ���Լ���3 mol��L��1 H2SO4��1 mol��L��1 NaOH��Һ��MnO2��KMnO4����������SO2��NaOH��Һ��Ũ���ᡢ��ɫʯ����Һ������NaCl��Һ��BaCl2��Һ��Ʒ����Һ����С����Ƶ�ʵ��װ��ͼ���£���B�м�����������SO2��NaOH��Һ��D�м���1 mol��L��1 NaOH��Һ����ش��������⣺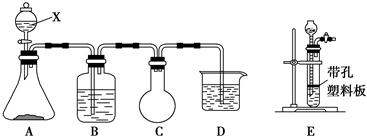
��. (1)д������X�����ƣ�________��
(2)д��A�з�����Ӧ�Ļ�ѧ����ʽ��__________________________��������Eװ�ô���
Aװ�õ�ԭ����__________________________________________��
(3)Cװ�õ�������_______________________________________��
(4)��С��ͨ����ʵ��Ҫ��֤Cl2��________(�Ư���ԡ�������ԭ�ԡ��������ԡ�)��
(5)��С����Ϊ��С����Ƶ�ʵ��װ����ȱ�ݣ����������С�����Ƹ�װ�á�����Ҫ��________��________(�A������B������C����D��)װ�ü�����һ��װ��________��ϴ��װ�á�
��.��С����Ϊ��Ӧһ��ʱ���Bװ����Һ(ǿ����)�п϶�����Cl����OH����SO42�������ܻ����������������ӡ�
�������ʵ�������С��̽��������Һ�п��ܴ��ڵ����������ӡ�
(1)�����������
����1��ֻ����________����ֻ����________��
����2���������߶�________(����ڡ������ڡ�)��
(2)�����ʵ����֤�������ȷ�ԣ�_____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��֪Cl2�ͼ���Һ�ڲ�ͬ�����£��õ��IJ��ﲻͬ��ij��ȤС������ͼ��ʾװ����ȡ����ء��������ƺ�̽����ˮ�����ʡ���3Cl2+6KOH KClO3+5KCl+3H2O ��
KClO3+5KCl+3H2O ��
ͼ�У�AΪ��������װ�ã�B���Թ���ʢ��15 mL 30% KOH��Һ��������ˮԡ�У�C���Թ���ʢ��15 mL 8% NaOH��Һ�������ڱ�ˮԡ�У�D���Թ��������ɫʯ����Һ������д���пհף�
��1����ȡ����ʱ����Բ����ƿ�����һ�������Ķ������̣�ͨ�� (����������)��Բ����ƿ�м���������Ũ���ᡣװ��A�з�Ӧ�����ӷ���ʽΪ ����Ҫ����ƿ�м����Ƭ�� ��ѡ�����Ҫ����������Ҫ������
��2����Ӧ��������ƿ�����Һ ��
Aһ�������ԣ�B���������ԣ�Ҳ����Ϊ���ԣ�Cֻ�������ԣ�Dֻ�л�ԭ�ԣ�E�������������л�ԭ��
��3����Ӧ��ϣ�B�Թ�����������������������ȴ���д�������������ͼ�з��ϸþ����ܽ�����ߵ��� (������ĸ)����B���Թ��з�����þ�������õ��IJ��������� ��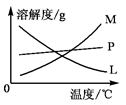
��4����С��ͬѧ�����Ƶõ�����ز���ƫ�ͣ����ܵ�һ��ԭ����Cl2�к���HCl���塣�Դ��������ͨ���Ľ�ʵ��װ�õķ������б��⡣������ ��
��5��ʵ���пɹ۲쵽D���Թ�����Һ����ɫ����ɫ�ȱ�Ϊ_________ɫ�����ձ�Ϊ________ɫ��
��6��Cװ���з�Ӧ��ϵ�������______________________________________________��
��7������װ��ͼ�����л���ȱ�ٵ�ʵ��װ�ã���ע���Լ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
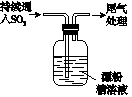
�ס�����ͬѧ����ͼ��ʾװ�ý���ʵ��,̽������������Ӧ�IJ��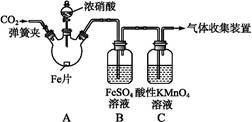
������:��.��Ũ��������ý�����Ӧ������,��������Ũ�ȵĽ���,�����ɵIJ�����+4��+2��-3�۵ȵ��Ļ����
��.FeSO4+NO Fe(NO)SO4(��ɫ)����H<0��
Fe(NO)SO4(��ɫ)����H<0��
��.NO2��NO���ܱ�KMnO4�������ա�
��ʵ������������¼����:
| ʵ����� | ʵ������ |
| ���ɼ�,ͨ��һ��ʱ��CO2,�رյ��ɼС� | |
| ��Һ©������,��Ũ���Ỻ��������ƿ��,�رջ����� | ���������� |
| ������ƿ,��Ӧ��ʼ��ֹͣ���ȡ� | ��A���к���ɫ�������,һ��ʱ���,������ɫ��dz;B����Һ����ɫ;C����Һ��ɫ��dz�� �ڷ�Ӧֹͣ��,A������ʣ�ࡣ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ȤС����Ʋ�����������ʵ����̽��Cl2��Ư�۵��Ʊ����й����ʡ�
��1��ʵ������������װ���Ʊ����﴿�����������밴������������������ķ��������������ӣ�
H�������������������������������������������������������ƿ���е��Լ�Ϊ��������������������������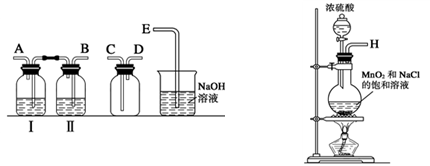
��2��д����ҵ����������ʯ������ȡƯ�۵Ļ�ѧ��Ӧ����ʽ��
������������������������������������������������������������������������������������������
ijѧ���������ʵ���һ��̽��SO2��Ư�۾��ķ�Ӧ��
| ���� | ���� |
| ȡ4 gƯ�۾����壬����100 mLˮ | ���ֹ����ܽ⣬��Һ������ɫ |
| ���ˣ���Ư�۾���Һ��pH | pH��ֽ�ȱ�����ԼΪ12��������ɫ |
 A | ��.Һ���Ϸ�������״�� ��.�Ժ��ֻ��ǣ���Һ��Ϊ����ɫ ��.�Ժ���������ɫ����������ɫ��ȥ |
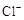 �ķ����ǣ��� ����������������������������������������
�ķ����ǣ��� �����������������������������������������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
I���������ᴿ����ʯ(��Ҫ������������)������ͼ��
��Ҫ��ش��������⣺
��1����������Ҫ��ʱ�ⶨpH����ʵ��������pH��ֽ�ⶨ��ҺpH�IJ�����______________________________��
��2������Fe3+����ȫ����Fe(OH)3�����ķ�����________________________________��
��3������ҺB�õ�����B������Ҫ�ɷ���__________��
II��ij��ѧС����ʵ������Ũ�����ͭ��Ӧ��ȡ����������̽�����������Ƿ���֧��ľ̿��ȼ�գ���ʵ��װ��ͼ���£�
��1���������������Ӹ������ӿڣ�˳��Ϊa��______��______��______��______��f��װ��C������________________��װ��D���Լ���__________________��
��2����֪����������̼�ڵ�ȼ�����·�Ӧ�����������ʵ�����Ϊ2��1�������壬��÷�Ӧ�Ļ�ѧ����ʽΪ��___________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com