
��֪R��CH==CHOH(ϩ��)���ȶ�,�ܿ�ת��ΪR��CH2CHO��
����������Ϣ�ش���������:
(1)A�ķ���ʽΪ_______________________;
(2)��Ӧ�ڵĻ�ѧ����ʽ��_______________________;
(3)A�Ľṹ��ʽ��_______________________;
(4)��Ӧ�ٵĻ�ѧ����ʽ��_______________________;
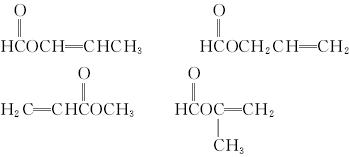
(5)A�ж���ͬ���칹��,д���ĸ�ͬʱ����(i)�ܷ���ˮ�ⷴӦ��(ii)��ʹ������Ȼ�̼��Һ��ɫ����������ͬ���칹��Ľṹ��ʽ��___________��____________��____________��____________��
��6��A����һ��ͬ���칹�壬�����������̼ԭ����һ��ֱ���ϣ����Ľṹ��ʽΪ_________��
��1��C4H6O2
(2)CH3CHO+2Cu(OH)2![]() CH3COOH+Cu2O+2H2O
CH3COOH+Cu2O+2H2O
(3)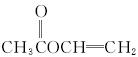
(4)
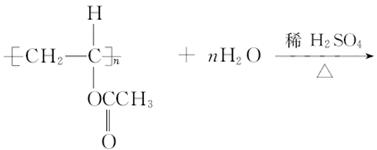
(5)
(������ȷ��Ҳ�ɣ�
��6��HOCH2��C�ԡ�C��CH2OH
����:�ɻ�����A����Է�������Ϊ86,̼����������Ϊ55.8%,��Ϊ7.0%,����Ϊ���ɼ��������ʽΪC4H6O2��A����ˮ��˵�����������������ֿ��Ծۺ�˵�����в�����̼̼˫��������ͼ���Կ���ˮ����������ת��������֪CDE��Ϊ2��̼ԭ�ӣ���DΪCH3COOH��AΪCH3COOCH====CH2��


 Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д� ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д� ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ������A��Է�������Ϊ86��̼����������Ϊ55.8%����Ϊ7.0%������Ϊ����A����ط�Ӧ��ͼ��ʾ��
������A��Է�������Ϊ86��̼����������Ϊ55.8%����Ϊ7.0%������Ϊ����A����ط�Ӧ��ͼ��ʾ��| �� |
| �� |












�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ������A��Է�������Ϊ86��̼����������Ϊ55.8%����Ϊ7.0%������Ϊ����A����ط�Ӧ��ͼ��ʾ����֪R-CH=CHOH��ϩ�������ȶ����ܿ�ת��ΪR-CH2CHO��
������A��Է�������Ϊ86��̼����������Ϊ55.8%����Ϊ7.0%������Ϊ����A����ط�Ӧ��ͼ��ʾ����֪R-CH=CHOH��ϩ�������ȶ����ܿ�ת��ΪR-CH2CHO��

| Cu |
| Cu |






�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
![]()
![]() ������A��Է�������Ϊ86��̼����������Ϊ55.8%����Ϊ7.0%������Ϊ����A����ط�Ӧ����ͼ��ʾ:
������A��Է�������Ϊ86��̼����������Ϊ55.8%����Ϊ7.0%������Ϊ����A����ط�Ӧ����ͼ��ʾ:
![]()
![]()

![]()
![]() ��֪R-CH=CHOH(ϩ��)���ȶ�,�ܿ�ת��Ϊ
��֪R-CH=CHOH(ϩ��)���ȶ�,�ܿ�ת��Ϊ![]() ��
��
![]()
![]() ����������Ϣ�ش���������:
����������Ϣ�ش���������:
![]()
![]() (1) A�ķ���ʽΪ ;
(1) A�ķ���ʽΪ ;
![]()
![]() (2) ��Ӧ�ڵĻ�ѧ����ʽ�� ;
(2) ��Ӧ�ڵĻ�ѧ����ʽ�� ;
![]()
![]() (3) A�Ľṹ��ʽ�� ;
(3) A�Ľṹ��ʽ�� ;
![]()
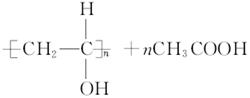
![]() (4) ��Ӧ�ٵĻ�ѧ����ʽ��
(4) ��Ӧ�ٵĻ�ѧ����ʽ��
![]()
![]() ;
;
![]()
![]() (5) A�ж���ͬ���칹��,д���ĸ�ͬʱ����(i)�ܷ���ˮ�ⷴӦ(ii)��ʹ������Ȼ�̼��Һ��ɫ����������ͬ���칹��Ľṹ��ʽ�� �� �� ��
(5) A�ж���ͬ���칹��,д���ĸ�ͬʱ����(i)�ܷ���ˮ�ⷴӦ(ii)��ʹ������Ȼ�̼��Һ��ɫ����������ͬ���칹��Ľṹ��ʽ�� �� �� ��
![]()
![]() ��
��
![]()
![]() ��6��A����һ��ͬ���칹�壬�����������̼ԭ����һ��ֱ���ϣ����Ľṹ��ʽΪ
��6��A����һ��ͬ���칹�壬�����������̼ԭ����һ��ֱ���ϣ����Ľṹ��ʽΪ ![]()
![]() ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012������ʡ�߶���ѧ�����п��Ի�ѧ���������� ���ͣ������
��11�֣�������A��Է�������Ϊ86��̼����������Ϊ55.8%����Ϊ7.0%��
����Ϊ����A����ط�Ӧ����ͼ��ʾ��
��֪R��CH��CHOH��ϩ�������ȶ����ܿ�ת��ΪR��CH2CHO������������Ϣ�ش��������⣺

��1��A�ķ���ʽΪ������������������������������������������������
��2����Ӧ�ڵĻ�ѧ����ʽ������������������������������������������
��3��A�Ľṹ��ʽ������������������������ ����������������������
��4����Ӧ�ٵĻ�ѧ����ʽ������������������������������������������
��5��A�ж���ͬ���칹�壬д���ĸ�ͬʱ���㣺
��i���ܷ���ˮ�ⷴӦ��
��ii����ʹ������Ȼ�̼��Һ��ɫ����������ͬ���칹��Ľṹ��ʽ��
������ �������� ����
������ ���������� �� ����
��6��A����һ��ͬ���칹�壬�����������̼ԭ����һ��ֱ���ϣ����Ľṹ��ʽΪ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com