
ijČÜŅŗÖŠæÉÄÜŗ¬ÓŠĻĀĮŠ5ÖÖĄė×ÓÖŠµÄij¼øÖÖ£ŗNa+”¢NH4+”¢Mg2+”¢Al3+”¢Cl”„”£ĪŖČ·ČĻøĆČÜŅŗ×é³É½ųŠŠČēĻĀŹµŃé£ŗ¢ŁČ”20.0 mLøĆČÜŅŗ£¬¼ÓČė25.0 mL 4.00 mol”¤L-1NaOHČÜŅŗ£¬ÓŠ°×É«³Įµķ”¢ĪŽŲŻ¼¤ĘųĪ¶ĘųĢ唣¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ£¬µĆ³Įµķ1.16 g”£ŌŁ½«ĀĖŅŗĻ”ŹĶÖĮ100 mL£¬²āµĆĀĖŅŗÖŠc(OH”„)ĪŖ0.20 mol”¤L-1£»¢ŚĮķČ”20.0 mLøĆČÜŅŗ£¬¼ÓČė×ćĮæµÄAgNO3ČÜŅŗ£¬Éś³É°×É«³Įµķ11.48 g”£ÓÉ“ĖæÉµĆ³ö¹ŲÓŚŌČÜŅŗ×é³ÉµÄÕżČ·½įĀŪŹĒ
| A£®Ņ»¶Øŗ¬ÓŠMg2+”¢Al3+”¢Cl”„£¬²»ŗ¬Na+”¢NH4+ |
| B£®Ņ»¶Øŗ¬ÓŠNa+”¢Mg2+”¢Cl”„£¬²»ŗ¬NH4+£¬æÉÄÜŗ¬ÓŠAl3+ |
| C£®c (Cl”„) ĪŖ 4.00 mol”¤L-1£¬c (Al3+) ĪŖ1.00 mol”¤L-1 |
| D£®c (Mg2+) ĪŖ 1.00 mol”¤L-1£¬c(Na+ ) ĪŖ 0.50 mol”¤L-1 |
D
½āĪöŹŌĢā·ÖĪö£ŗøł¾ŻŹµŃé¢ŁÅŠ¶Ļ£¬ŌČÜŅŗÖŠŅ»¶ØÓŠMg2+£¬ĪŽNH4+”£²Ī¼Ó·“Ó¦µÄOH”„£ŗ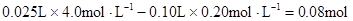 £¬n(Mg2+)=
£¬n(Mg2+)=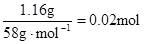 Mg2+ + 2OH”„= Mg(OH)2”ż£¬ÓėMg2+·“Ó¦µÄOH”„£ŗ
Mg2+ + 2OH”„= Mg(OH)2”ż£¬ÓėMg2+·“Ó¦µÄOH”„£ŗ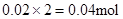 £¬ĖłŅŌŌČÜŅŗÖŠŅ»¶Ø“ęŌŚAl3+”£ÓėAl3+·“Ó¦µÄOH”„£ŗ
£¬ĖłŅŌŌČÜŅŗÖŠŅ»¶Ø“ęŌŚAl3+”£ÓėAl3+·“Ó¦µÄOH”„£ŗ £¬ŅņĀĖŅŗÖŠ»¹ÓąOH”„£¬Ōņ·¢Éś·“Ó¦ Al3+ + 4OH”„= AlO2”„ + 2H2O £¬n(Al3+ )=
£¬ŅņĀĖŅŗÖŠ»¹ÓąOH”„£¬Ōņ·¢Éś·“Ó¦ Al3+ + 4OH”„= AlO2”„ + 2H2O £¬n(Al3+ )= 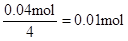 £»ÓÉŹµŃé¢Ś½įŗĻÉĻĆęµÄ¼ĘĖ揿¾Ż£¬µĆ n(Cl”„)=
£»ÓÉŹµŃé¢Ś½įŗĻÉĻĆęµÄ¼ĘĖ揿¾Ż£¬µĆ n(Cl”„)= 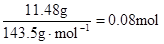 £» n(Cl”„)£¾2n(Mg2+) + 3n(Al3+ ) ĖłŅŌŌČÜŅŗÖŠ»¹ŗ¬ÓŠ Na+ £»2n(Mg2+) + 3n(Al3+ )+ n(Na+ ) =
£» n(Cl”„)£¾2n(Mg2+) + 3n(Al3+ ) ĖłŅŌŌČÜŅŗÖŠ»¹ŗ¬ÓŠ Na+ £»2n(Mg2+) + 3n(Al3+ )+ n(Na+ ) = 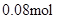 n(Na+ ) =
n(Na+ ) = 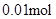
ŌČÜŅŗÖŠĄė×ÓµÄÅØ¶Č£ŗ
c (Cl”„) = 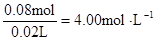 c (Al3+)=
c (Al3+)=
c (Mg2+)=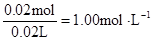 c(Na+ )=
c(Na+ )= 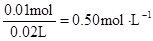
¹ŹŃ”D”£
æ¼µć£ŗ±¾Ģāæ¼²éѧɜ³£¼ūĄė×ӵļģŃé·½·ØŗĶĄė×ÓÄŃ¶ČµÄ¼ĘĖć£¬æÉŅŌøł¾ŻĖłŃ§µÄÖŖŹ¶Ą“»Ų“š£¬ÄѶČÖŠ”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
£Ø1£©ĪżĪŖµŚ¢ōA×åŌŖĖŲ£¬ĪżµÄµ„ÖŹŗĶ»ÆŗĻĪļÓėijŠ©ĪļÖŹµÄ»ÆѧŠŌÖŹÓŠŠķ¶ąĻąĖĘÖ®“¦”£ŅŃÖŖĪżŌŖĖŲ¾ßÓŠČēĻĀŠŌÖŹ£ŗ £»
£»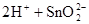



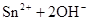 ӣ
ӣ
ŹŌ»Ų“š£ŗ
¢ŁĪżČÜÓŚŃĪĖį£¬ŌŁĻņ·“Ó¦ŗóµÄČÜŅŗÖŠĶØČėĀČĘų£¬ÓŠ¹Ų·“Ó¦ĄąĖĘÓŚĢśµÄĻąÓ¦±ä»Æ£¬ŹŌŠ“³öÓŠ¹Ų·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ_____________________________________________________£¬_______________________________________”£
¢Ś½«¢ŁÖŠČÜŅŗÕōøÉŗó¼ĢŠų×ĘÉÕĖłµĆ¹ĢĢ壬±ä»Æ¹ż³ĢĄąĖĘÓŚFeCl3ČÜŅŗĻąÓ¦µÄ±ä»Æ£¬Ōņ×īŗóµĆµ½µÄ¹ĢĢåĪļÖŹŹĒ__________£ØĢī»ÆѧŹ½£©”£
¢ŪČōæÉÓĆSnCl2ČÜŅŗÓė¹żĮæµÄ¼īČÜŅŗ·“Ó¦µÄ·½·ØÖĘSn£ØOH£©2£¬øĆ¼īæÉŃ”ÓĆ________”£
£Ø2£©Ä³ĪŽÉ«Ļ”ČÜŅŗXÖŠ£¬æÉÄÜŗ¬ÓŠĻĀ±ķĖłĮŠĄė×ÓÖŠµÄij¼øÖÖ”£
| ŅõĄė×Ó | 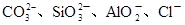 |
| ŃōĄė×Ó |  |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠø÷×éĄė×ÓŌŚČÜŅŗÖŠÄÜ“óĮæ¹²“ęµÄŹĒ
A£®Na£«”¢Al3£«”¢Cl£”¢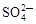 | B£®Cu2£«”¢Cl£”¢ ”¢OH£ ”¢OH£ |
C£®Ca2£«”¢Na£«”¢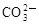 ”¢ ”¢ | D£®H£«”¢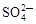 ”¢ ”¢ ”¢OH£ ”¢OH£ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠĄė×Ó·½³ĢŹ½ŹéŠ“ÕżČ·µÄŹĒ£Ø £©
| A£®ĻņµĪÓŠ·ÓĢŖµÄ¹čĖįÄĘČÜŅŗÖŠ±ß¼Ó±ßÕńµ“µŲµĪ¼ÓŃĪĖįÖĮŗģÉ«±äĒ³²¢½Ó½üĻūŹ§ 2H++SiO32”Ŗ=H2SiO3£Ø½ŗĢ壩 |
B£®ÓƶčŠŌµē¼«µē½āMgCl2ČÜŅŗ£ŗ2Cl”Ŗ+2H2O Cl2”ü+H2”ü+2OH”Ŗ Cl2”ü+H2”ü+2OH”Ŗ |
C£®Ģ¼ĖįĒāļ§ČÜŅŗÓė×ćĮæµÄNaOHČÜŅŗ»ģŗĻŗó¼ÓČČ£ŗ NH4£«£«OH£ NH3”ü£«H2O NH3”ü£«H2O |
| D£®5£®6 g FeÓė200 mL 2£®0 mol/L HNO3ČÜŅŗ³ä·Ö·“Ó¦£ŗ3Fe + 2NO3”Ŗ+ 8H+£½3Fe2+ + 2NO”ü + 4H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
³£ĪĀ£¬ĻĀĮŠø÷×éĄė×ÓŌŚÖø¶ØČÜŅŗÖŠÄÜ“óĮæ¹²“ęµÄŹĒ( )
| A£®pH=1µÄČÜŅŗÖŠ: I-”¢NO3£”¢SO42£”¢Na£« |
| B£®ÓÉĖ®µēĄėµÄc(H+)=1”Į10-14 mol”¤L-1µÄČÜŅŗÖŠ£ŗCa2£«”¢K£«”¢Cl£”¢HCO3£ |
| C£®c(H+)£Æc(OH£)=1012µÄČÜŅŗÖŠ£ŗ NH4£«”¢Al3£«”¢NO3£”¢Cl£ |
| D£®c(Fe3£«)="0.1" mol”¤L-1µÄČÜŅŗÖŠ£ŗK£«”¢ClO£”¢SO42£”¢SCN£ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ČōČÜŅŗÖŠÓÉĖ®µēĄė²śÉśµÄc(OH£)£½1”Į10£14 mol”¤L£1£¬Āś×ć“ĖĢõ¼žµÄČÜŅŗÖŠŅ»¶ØæÉŅŌ“óĮæ¹²“ęµÄĄė×Ó×éŹĒ( )
| A£®Al3£« Na£« NO3£ Cl£ | B£®K£« Na£« Cl£ NO3£ |
| C£®K£« Na£« Cl£ AlO2£ | D£®K£« NH4£« SO42£ NO3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠø÷×éÖŠĮ½ÖÖĪļÖŹŌŚČÜŅŗĄļµÄ·“Ó¦£¬æÉÓĆĶ¬Ņ»Ąė×Ó·½³ĢŹ½±ķŹ¾µÄŹĒ
| A£®KCl+AgNO3£»AlCl3+ AgNO3 |
| B£®NaHCO3+H2SO4£»Na2CO3+HCl |
| C£®NaHCO3+NaOH£»Ca£ØHCO3£©+KOH |
| D£®BaCl2+H2SO4£»Ba(OH)2+H2SO4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ÄÜÕżČ·±ķŹ¾ĻĀĮŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ
| A£®Ģ¼ĖįĒāÄĘČÜŅŗÖŠµĪČėĒāŃõ»ÆøĘČÜŅŗ£ŗHCO3ØD + OHØD £½ CO32ØD£« H2O |
| B£®¶žŃõ»ÆĮņĶØČė“ĪĀČĖįÄĘČÜŅŗ£ŗSO2 + ClOØD + 2OHØD£½ SO42ØD£«ClØD£«H2O |
| C£®Įņ»Æ±µ¼ÓČėĻ”ĮņĖį£ŗBaS + 2H£«£½ H2S”ü+ Ba2+ |
| D£®¹čĖįÄĘČÜŅŗÓė“×ĖįČÜŅŗ»ģŗĻ£ŗSiO32£+2H£«£½H2SiO3”ż |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠĄė×Ó·½³ĢŹ½ÕżČ·µÄŹĒ
| A£®°±Ė®ĪüŹÕ×ćĮæµÄSO2ĘųĢå£ŗOH££«SO2£½HSO3£ |
| B£®½«ĶĖæĶ¶ČėĻ”ĻõĖįÖŠ£ŗCu£«4H£«£«NO3££½Cu2£«£«NO2”ü£«2H2O |
| C£®½«H2O2µĪČėĖįŠŌKMnO4ČÜŅŗÖŠ£ŗ2MnO4£+10H++3H2O2£½2Mn2++3O2”ü+8H2O |
| D£®NaHSO4ČÜŅŗÖŠ¼ÓČė¹żĮæBa(OH)2ČÜŅŗ£ŗH£«£«SO42££«Ba2£«£«OH££½BaSO4”ż£«H2O |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com