

 ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д� Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ������ͼ��ʾ�������ڢ����ֲ�ͬ�Ļ����У�������ʴ�������ɴ�С��˳���ǣ�����ţ�
������ͼ��ʾ�������ڢ����ֲ�ͬ�Ļ����У�������ʴ�������ɴ�С��˳���ǣ�����ţ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ����� | �� | �� | �� |
| ʵ�� ���� |
 |
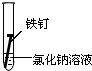 |
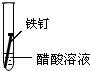 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���˽̰���л�ѧѡ��1 3.2�����ĸ�ʴ�ͷ�����ϰ���������棩 ���ͣ������
�ݱ�����ȫ����ÿ���������ʴ��ɵ�ֱ�Ӿ�����ʧԼ��7000����Ԫ���ҹ��������ʴ��� ����ʧռ����������ֵ��GNP����4%��
��1��������ݲ�ͬԭ��,�����ֹ������ʴ������������ʩ��
�� ��
�� ��
��2����д����ϡ�����ȥ����Ļ�ѧ��Ӧ����ʽ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com