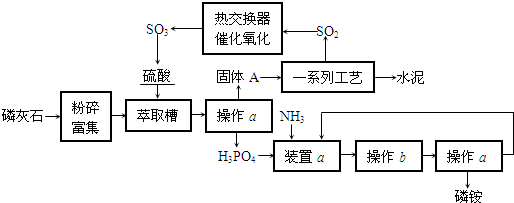
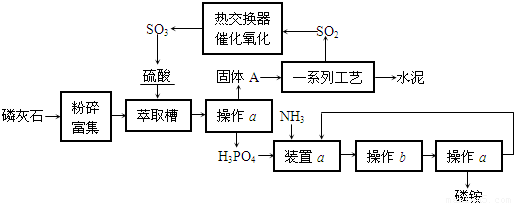
�⣺��1���ü��ȹ���ķ����Ʊ��������а����������ȣ�����Cװ�ÿ�֪������������ˮ������Fװ�ÿ�֪�ռ��������������ſ����������������ܶ�С�ڿ����������Ʊ�������Ӧ���ǰ�����ʵ�����Ʊ������ķ���ʽ�ǣ�2NH
4Cl+Ca��OH��
2
CaCl
2+2NH
3��+2H
2O���ʴ�Ϊ��2NH
4Cl+Ca��OH��
2
CaCl
2+2NH
3��+2H
2O��
��2�������Ƶõİ����к���ˮ����������Ҫ�������и��������B�з��ü�ʯ�ң���B����ﰱ�������ã��ʴ�Ϊ�����ﰱ����
��3�����Ը��ݰ�����ˮ��Һ�Լ��Լ��鰱��������ˮ��ʹ��ɫʯ����ֽ����ɫ���䷽��Ϊ����ʪ��ĺ�ɫʯ����ֽ����a��������ֽ��Ϊ��ɫ����F���ѳ������������ڰ�����Ũ����������̣����Ҳ�������·������ò�����պȡŨ�����a������������̣���F�г����������ʴ�Ϊ����ʪ��ĺ�ɫʯ����ֽ����a��������ֽ��Ϊ��ɫ����F�е��ѳ������������ò�����պȡŨ�����a������������̣���F�г�����������
��4����Cװ���õ��õ�©������ֹ���������ã�����ֹ�����������ʴ�Ϊ����ֹ������������
��5���ر�K2��K4����K1����Ҫʹ��ƿ�еİ�����ˮ�Ӵ�������ʹ��������ˮ����ѹ�����С���Ӷ�������Ȫ��Ҫ�ﵽ���Ŀ�ģ�����Ҫ�ر�K5����K3�����ּ�ѹ����ƿD��ʹ���쵼���е�ˮ���������백���Ӵ�����������K5����ʹҺ���������ϵ�������ƿ���������跨������ƿ����ѹ���Ѳ��ְ���ͨ�����쵼���ų����Ӷ�������Ӧ����������ë��������ƿ�������ر�K5����K3�����ἷѹ����ƿD��Ȼ���K5�������ë��������ë������ƿ��ס��
����ƿ�����ΪVL������ƿ�а�ˮ��Ũ��Ϊ

=0.045mol/L��
�ʴ�Ϊ���ر�K5����K3�����ἷѹ����ƿD��Ȼ���K5�����K1��K5�����֣�����ë��������ë������ƿ��ס����
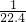
mol/L����0.045mol/L����
�����������ʴ�Ϊ��
��������1������װ������ȡ���ռ������жϳ��Ʊ����ǰ�����Ȼ������д��ѧ����ʽ��
��2�����ݰ����л���ˮ����˼����
��3�����ݰ����Ļ�ѧ����ȥ������
��4������װ�õ��ص㣬������©����������
��5������������Ȫʵ��ķ���˼�����������ʵ���Ũ�ȵļ��㹫ʽ���㣮
����������Ҫ���Ʊ�������ռ�װ�õ�������������Ҫ�Ʊ��������ǰ����������ݰ���������Ȫʵ��Ļ�����̽��������Ȫʵ��ķ�����

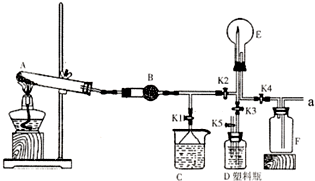 ��ͼ��ʵ������ij��������Ʊ������ʼ��鼰�ռ�װ��ͼ��
��ͼ��ʵ������ij��������Ʊ������ʼ��鼰�ռ�װ��ͼ�� CaCl2+2NH3��+2H2O���ʴ�Ϊ��2NH4Cl+Ca��OH��2
CaCl2+2NH3��+2H2O���ʴ�Ϊ��2NH4Cl+Ca��OH��2 CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O�� =0.045mol/L��
=0.045mol/L��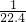 mol/L����0.045mol/L����
mol/L����0.045mol/L����