
��һ��NaCl��Na2CO3·10H2O��NaHCO3�Ļ���ijͬѧ�����ͼʵ�飬ͨ��������Ӧǰ��C��Dװ�������ı仯���ⶨ�û�����и���ֵ�����������
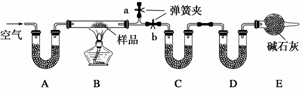
(1)����ǰͨ�������Ŀ����_______________________________________________��
��������Ϊ_______________________________________________________________
________________________________________________________________________��
(2)װ��A��C��D��ʢ�ŵ��Լ��ֱ�Ϊ��A______________��C______________��D________________��
(3)����Aװ�û���ʢ��NaOH��Һ��ϴ��ƿ�����õ�NaCl�ĺ�����________(�ƫ�ߡ�����ƫ�͡�����Ӱ�족����ͬ)����B�з�Ӧ���Ҳ���ˮ������������ⶨ�����NaHCO3�ĺ�����________������ȥEװ�ã�����Na2CO3·10H2O �ĺ�����________��
(4)����Ʒ����Ϊw g����Ӧ��C��D���ӵ������ֱ�Ϊm1 g��m2 g���ɴ˿�֪�������NaHCO3����������Ϊ_______________________________________________________
(�ú�w��m1��m2�Ĵ���ʽ��ʾ)��
�𰸡�(1)��ȥװ���е�ˮ�����Ͷ�����̼���ر�b����a������ͨ�������ֱ��a�������Ŀ�������ʹ����ʯ��ˮ�����Ϊֹ
(2)��ʯ�ҡ���ˮ����ͭ(����ˮCaCl2��P2O5��)����ʯ��
(3)ƫ�͡���Ӱ�졡ƫ��
(4)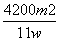 %
%
������(2)A��C��D����U�ιܣ�����ʢҺ���Լ���ֻ��ʢ�����Լ���A���Լ�������ͬʱ����CO2��ˮ��������A��ʢ���Ǽ�ʯ�ң�C��D�����ֱ�����ˮ������CO2����C����ʢ��ˮ����ͭ(����ˮCaCl2��P2O5��)��D����ʢ��ʯ�ҡ�
(3)��Aװ��ʢ��NaOH��Һֻ����CO2��������ˮ�������������в���ˮ������Na2CO3·10H2O��NaHCO3���������ڼ����л�������˲�õ�NaCl�ĺ�����ƫ�ͣ���B�з�Ӧ���Ҳ���ˮ������������ˮ������������С��Na2CO3·10H2O��NaHCO3���������ڼ����л��С������NaHCO3�������Ǹ���CO2���������м��㣬���Բ�õ�NaHCO3�ĺ�������Ӱ�죻����ȥEװ�ã���Dװ�ÿ����������������е�CO2��ʹ��NaHCO3�������ڼ����л����ʲ�õ�Na2CO3·10H2O�ĺ�����ƫ�͡�
(4)NaHCO3������������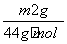 ��2��84 g·mol��1��w g��100%��
��2��84 g·mol��1��w g��100%��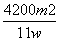 %��
%��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
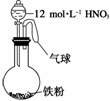 ��ͼ��ʾ����һ�����������м���һ�����12 mol·L��1�����ᣬ���ȳ�ַ�Ӧ������������ϵ��һ���������ڵ��� (����)
��ͼ��ʾ����һ�����������м���һ�����12 mol·L��1�����ᣬ���ȳ�ַ�Ӧ������������ϵ��һ���������ڵ��� (����)
��NO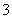 ����Fe3������H������NO ��NO2
����Fe3������H������NO ��NO2
A���� B���٢�
C���ڢܢ� D���٢ڢۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
[��ѧ��ѡ��2��ѧ�뼼��]��15�֣�
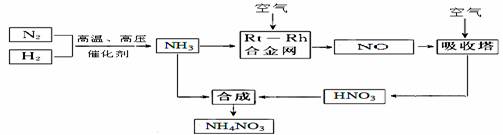 ��������ִ�ũҵ���������ж�ռ����Ҫ��λ����ͼ��������Ȼ���������ϳ�����淋ļ�Ҫ�����������̣�
��������ִ�ũҵ���������ж�ռ����Ҫ��λ����ͼ��������Ȼ���������ϳ�����淋ļ�Ҫ�����������̣�
�ش��������⣺
��1��N2�ĵ���ʽ ���ϳɰ��ķ�Ӧ�У�������1g���ų�����a KJ,д���÷�Ӧ���Ȼ�ѧ����ʽ ��
��2�����������Ļ�ѧ����ʽ�� ��
�Դӻ�ѧ��Ӧ���ʺͻ�ѧƽ��ԭ�������¶ȡ�ѹǿ�Ժϳɰ���Ӧ��Ӱ��
��
��3��������NH3�Ĺܵ�ij������й©�����ļ���
��
��4��ũҵ�����ϳ��ڡ�����ʹ�û�������泥�����������ữ��ԭ���ǣ������ӷ���ʽ�ش� ��
��5��25��ʱ����x mol NH4NO3����һ����ˮ�У������Һ�еμ�y L��ˮ����Һ�����ԣ���μӰ�ˮ������ˮ�ĵ���ƽ�⽫ ������� �������������ƶ������μӰ�ˮ�����ʵ���Ũ��Ϊ ��25��ʱ��Kb(NH3��H2O)=2.0 �� 10-5 mol��L-1����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ж�������ȷ�Ļ����̡�������Ļ�������
(1)1 mol�κ����ʶ�����6.02��1023������ (����)
(2)1 molˮ�к���2 mol���1 mol�� (����)
(3)NaOH��Ħ������Ϊ40 g (����)
(4)1 mol O2��������������Է���������� (����)
(5)������Ħ������(��λg·mol��1)����ֵ�ϵ����������ԭ������ (����)
(6)2 mol H2O��Ħ��������1 mol H2O��Ħ��������2�� (����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NAΪ�����ӵ����������a gij��̬˫ԭ�ӷ��ӵķ�����Ϊp����b g�������ڱ�״���µ����V(L)�� (����)
A.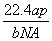 B.
B.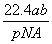
C.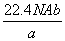 D.
D.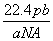
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й���ͬ��ͬѹ�µ���������12C18O��14N2���ж���ȷ����
(����)
A��������ʱ�ܶ����
B��ԭ�������ʱ���е����������
C��������ʱ���еĵ��������
D���������ʱ���е����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ӵ�������NA��ʾ������������ȷ���� (����)
A������ʱ��0.1 L 0.1 mol·L��1��������Һ��ˮ�������H����Ϊ10��13NA
B��1 L 0.2 mol·L��1 MgSO4��Һ�е���������С�� 0.2NA
C��2.88 g ND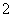 �к��еĵ�������1.8NA
�к��еĵ�������1.8NA
D�����³�ѹ�£�3 g HCHO��CH3COOH������к���0.4NA��ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ҫ����ij�������е���Ԫ�أ���ȷ��ʵ�鷽����
A��������ˮ���۲�ˮ���Ƿ����غ�ɫ����
B������AgNO3��Һ���ټ���ϡ��������ԣ��۲�����dz��ɫ��������
C������NaOH��Һ���ȣ�Ȼ��ȡ�ϲ���ҹ������ϡ��������ԣ��ٵ���AgNO3��Һ���۲�����dz��ɫ��������
D������NaOH��Һ���ȣ���ȴ�����AgNO3��Һ���۲�����dz��ɫ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪����A�Ƿ����廯���A�����б�������2��ȡ������������֧����A�ĺ˴Ź���
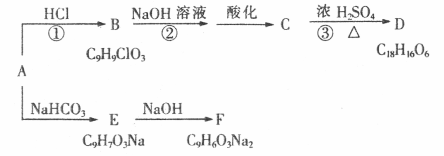 ������6�����շ壬�������Ϊ1:1:1:1:2:2���ܹ���FeCl3��Һ������ɫ��Ӧ��D�����г���2�����������1����Ԫ�������ǵ�ת����ϵ���£�
������6�����շ壬�������Ϊ1:1:1:1:2:2���ܹ���FeCl3��Һ������ɫ��Ӧ��D�����г���2�����������1����Ԫ�������ǵ�ת����ϵ���£�
 | |||
 | |||
��ش��������⣺
(1)A�����������ŵ�����Ϊ ��
(2)��Ӧ�١������ڼӳɷ�Ӧ�� �� ������ţ���
(3)B�Ľṹ��ʽ ��D�Ľṹ��ʽ ��
(4)A��E�Ļ�ѧ����ʽΪ ��
(5)��������������A��ͬ���칹���� �֡�
�ٷ����廯��������ϵ�ȡ����������3����
������FeCl3��Һ������ɫ��Ӧ�Ҳ���ˮ�⣻
��lmol�����ʿ���4mol�zAg(NH3)2�{+����������Ӧ��
��lmol�����ʿ���5molH2�ӳɡ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com