
����Ŀ����ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
![]() ��Ũ��������ʵ���Ũ��Ϊ ______
��Ũ��������ʵ���Ũ��Ϊ ______ ![]() ��
��
![]() ȡ����������ĸ�������Һʱ�������������в�����ȡ����Ķ��ٶ��仯���� ______ ��
ȡ����������ĸ�������Һʱ�������������в�����ȡ����Ķ��ٶ��仯���� ______ ��
A.��Һ��HCl�����ʵ���![]() ��Һ��Ũ��
��Һ��Ũ��
C.��Һ��![]() ����Ŀ
����Ŀ![]() ��Һ���ܶ�
��Һ���ܶ�
![]() ijѧ����������Ũ���������ˮ����450mL���ʵ���Ũ��Ϊ
ijѧ����������Ũ���������ˮ����450mL���ʵ���Ũ��Ϊ![]() ϡ���ᣮ
ϡ���ᣮ
![]() ��ѧ����Ҫ��ȡ ______ mL����Ũ����������ƣ�
��ѧ����Ҫ��ȡ ______ mL����Ũ����������ƣ�
![]() ����ʱ������ȷ�IJ���˳����
����ʱ������ȷ�IJ���˳����![]() ����ĸ��ʾ��ÿ����ĸֻ����һ��
����ĸ��ʾ��ÿ����ĸֻ����һ��![]() ______ ��
______ ��
A.��30mLˮϴ���ձ�![]() �Σ�ϴ��Һ��ע������ƿ����
�Σ�ϴ��Һ��ע������ƿ����
B.����Ͳȷ��ȡ����Ũ���������������ر���ע��ʢ������ˮ![]() Լ
Լ![]() ���ձ��У��ò���������������ʹ���Ͼ���
���ձ��У��ò���������������ʹ���Ͼ���
C.������ȴ�������ز�����ע��500mL������ƿ��
D.������ƿ�ǽ����ߵ�ҡ��
E.���ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�������
F.����������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶���![]() ��
��
![]() �����ƹ����У�����ʵ������������Ƶ�ϡ��������ʵ���Ũ���к�Ӱ�죿
�����ƹ����У�����ʵ������������Ƶ�ϡ��������ʵ���Ũ���к�Ӱ�죿![]() �ƫ�ߡ���ƫ�͡�����Ӱ�족
�ƫ�ߡ���ƫ�͡�����Ӱ�족![]() ��
��
I������Ͳ��ȡŨ����ʱ���ӹ۲찼Һ�� ______
II������Ͳ��ȡŨ�����ϴ����Ͳ![]() �Σ�ϴ��ҺҲת�Ƶ�����ƿ ______
�Σ�ϴ��ҺҲת�Ƶ�����ƿ ______
III����Һע������ƿǰû�лָ������¾ͽ��ж��� ______
![]() ���ڱ�״���£���VLHCl��������1Lˮ�У�������Һ�ܶ�Ϊd
���ڱ�״���£���VLHCl��������1Lˮ�У�������Һ�ܶ�Ϊd![]() �������Һ�����ʵ���Ũ��Ϊ ______
�������Һ�����ʵ���Ũ��Ϊ ______ ![]() ����ĸ
����ĸ![]()
A.![]() B.
B.![]() C.
C.![]() D.
D.![]()
���𰸡� 12 BD ![]() BCAFED ƫ�� ƫ�� ƫ�� A
BCAFED ƫ�� ƫ�� ƫ�� A
����������1���������ʵ���Ũ�����������������Ļ��㹫ʽ���㡣
��2��ȡ����������ĸ�������Һ����Һ��Ũ�ȡ���Һ���ܶȲ�����ȡ����Ķ��ٶ��仯��HCl���ʵ�����Cl-����Ŀ��������Ķ����йء�
��3��������c��Ũ��Һ��V��Ũ��Һ��=c��ϡ��Һ��V��ϡ��Һ��������
���������ʵ���Ũ����Һ��ʵ�鲽��Ϊ�����㡢����������ȡ�����ܽ⣨��ϡ�ͣ�����ȴ�����¡�ת�ơ�ϴ�ӡ����������ݡ���תҡ�ȡ�װƿ����ǩ��
�����ݹ�ʽcB=![]() ������������
������������
��4����HCl���������HCl���ʵ���������������Һ���������ܶȼ�����Һ�������������c��HCl��=![]() �����������ʵ���Ũ�ȡ�
�����������ʵ���Ũ�ȡ�
��1�����ʵ���Ũ�����������������Ļ��㹫ʽΪc=![]() �����Ũ�������ʵ���Ũ��Ϊ
�����Ũ�������ʵ���Ũ��Ϊ![]() =12mol/L��
=12mol/L��
��2����Һ�Ǿ�һ���ȶ��Ļ���ȡ����������ĸ�������Һ����Һ��Ũ�ȡ���Һ���ܶȲ�����ȡ����Ķ��ٶ��仯��HCl���ʵ�����Cl-����Ŀ��������Ķ����й�����ѡBD��
��3��������450mL��ҺӦѡ��500mL����ƿ������c��Ũ��Һ��V��Ũ��Һ��=c��ϡ��Һ��V��ϡ��Һ������ȡ��Ũ��������Ϊ![]() =12.5mL��
=12.5mL��
���������ʵ���Ũ����Һ��ʵ�鲽��Ϊ�����㡢����������ȡ�����ܽ⣨��ϡ�ͣ�����ȴ�����¡�ת�ơ�ϴ�ӡ����������ݡ���תҡ�ȡ�װƿ����ǩ������ʱ����ȷ�IJ���˳��ΪBCAFED��
�����ݹ�ʽcB=![]() ������
������
I.����Ͳ��ȡŨ����ʱ���ӹ۲찼Һ�棬����ȡ��Ũ�������ƫ�ͣ�n��HCl��ƫ�ͣ������Ƶ�ϡ��������ʵ���Ũ��ƫ�͡�
II.����Ͳ��ȡŨ�����ϴ����Ͳ2~3�Σ�ϴ��ҺҲת�Ƶ�����ƿ�У�n��HCl��ƫ�ߣ������Ƶ�ϡ��������ʵ���Ũ��ƫ�ߡ�
III.Ũ����ϡ��ʱʱ���ȣ���Һע������ƿǰû�лָ������¾ͽ��ж��ݣ�����ϡ��Һ���ƫ�ͣ������Ƶ�ϡ��������ʵ���Ũ��ƫ�ߡ�
��4��n��HCl��=![]() =
=![]() mol��m����Һ��=m��HCl��+m��H2O��=
mol��m����Һ��=m��HCl��+m��H2O��=![]() mol
mol![]() 36.5g/mol+1g/mL
36.5g/mol+1g/mL![]() 1000mL=
1000mL=![]() g��V����Һ��=
g��V����Һ��=![]() g
g![]() dg/mL=
dg/mL=![]() mL=
mL=![]() 10-3L������Һ���ʵ���Ũ��Ϊ
10-3L������Һ���ʵ���Ũ��Ϊ![]() mol
mol![]() ��
��![]() 10-3L��=
10-3L��=![]() mol/L����ѡA��
mol/L����ѡA��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������̼�ᶼ�dz������ᡣ
��1��̼��ĵ��뷽��ʽΪ__________________________________________________��
��2����0.1 mol��L��1 CH3COOH��Һ�м�ˮϡ�ͣ�c��CH3COO���� / c��CH3COOH���ı�ֵ��______�����������䡱��С������
��3��������ʵһ����˵��CH3COOH��������ʵ���____________������ĸ����
A. ��ͬ�¶��£�Ũ�Ⱦ�Ϊ1 mol��L��1������ʹ���ĵ����ԶԱȣ��������
B. 1 mol��L��1 CH3COOH��Һ��ʹ��ɫʯ����Һ���
C. 25��ʱ��1 mol��L��1 CH3COOH��Һ��pHԼΪ2
D. 10 mL 1mol��L��1��CH3COOH��Һǡ����10 mL 1mol��L��1 NaOH��Һ��ȫ��Ӧ
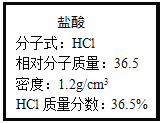
��4�������£���25 mL����������Һ����μ���0.2 mol/L������Һ���ζ���������ͼ��ʾ��
�ٸ�����������Һ�����ʵ���Ũ��Ϊ________mol��L��1
����B�㣬a________12.5 mL(����ڡ�����С�ڡ����ڡ�)�������NaOHǡ����ȫ��Ӧ����Һ��______����ᡱ����ԣ�ԭ����______________________�������ӷ���ʽ��ʾ������ʱ��Һ�и�����Ũ�ȴ�СΪ__________________________��
��A��B��C��D�����У�ˮ�ĵ���̶�������____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
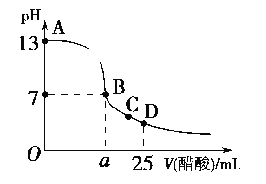
����Ŀ����֪��Ӧ��2NO2������ɫ��![]() N2O4����ɫ����H��0����һ������NO2����ע�����в��ܷ⣬�ı����λ�õĹ����У�����������ʱ��ı仯��ͼ��ʾ��������ɫԽ�����ԽС��������˵������ȷ���ǣ� ��
N2O4����ɫ����H��0����һ������NO2����ע�����в��ܷ⣬�ı����λ�õĹ����У�����������ʱ��ı仯��ͼ��ʾ��������ɫԽ�����ԽС��������˵������ȷ���ǣ� ��
A. b��ﵽƽ��״̬
B. b����a����ȣ�c��NO2����c��N2O4������С
C. d�㣺v��������v������
D. ����c�㽫�¶Ƚ��ͣ������ʽ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ѫ֬��һ�ֳ�������Ѫ�ܼ��������Ƹ�Ѫ֬����ҩI�ĺϳ�·�����£�
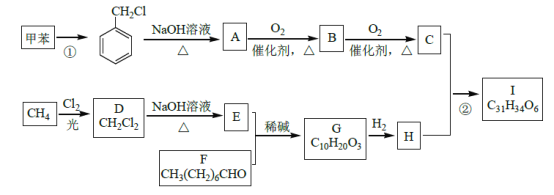
��֪��
�ش��������⣺
��1����Ӧ�������Լ��������ֱ���____________��F �Ļ�ѧ����Ϊ____________��
��2���ڵķ�Ӧ������______________��A��B �Ļ�ѧ����ʽΪ_________________��
��3��G �Ľṹ��ʽΪ______________��H �����������ŵ�������____________��
��4��������W ����Է��������Ȼ�����C ��14������������������W �Ŀ��ܽṹ��__�֡�
����FeCl3 ��Һ����ɫ�����ڷ����廯������ܷ���������Ӧ���к˴Ź���������ʾ��5 �ֲ�ͬ��ѧ�������⣬�������Ϊ2:2:2:1:1��д������Ҫ���W �Ľṹ��ʽ____________��
��5������üױ�����ȩΪԭ���Ʊ� �ĺϳ�·�ߣ��������Լ���ѡ���ϳ�·�߳��õı�ʾ��ʽΪ��
�ĺϳ�·�ߣ��������Լ���ѡ���ϳ�·�߳��õı�ʾ��ʽΪ�� ��____________��
��____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ������������ԭ��Ӧ����ˮ�Ȳ����������ֲ�����ԭ������
A.H2O��CaO��Ca(OH)2
B.2H2O��2F2��4HF��O2
C.2H2O![]() O2����2H2��
O2����2H2��
D.3NO2+ H2O��2HNO3+NO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ֻ��һ���Լ��Ϳ��Լ���������Һ����������Һ��������Һ�������Լ��ǣ� ��
A. NaOH��ҺB. Cu(OH)2����ҺC. ��ˮD. Na2CO3��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��AG��lgc(H+)/c(OH-)����������0.01mol��L1 NH3��H2O��Һ�ζ�20.00mL 0.01mol��L1ijһԪ��HA���ɵ���ͼ��ʾ�Ľ��������˵���д������
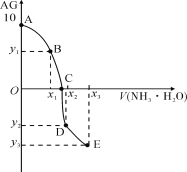
A. �õζ�ʵ�����ѡ�ü�����ָʾ��
B. ���������У�C��ʱˮ�ĵ���̶����
C. ��x3��30������3c(OH)��c(NH4+)+3c(H+)��2c(NH3��H2O)
D. A��C�Ĺ����У��ɴ��ڣ�c(A)��c(H+)��c(NH)��c(OH)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����A��B��C��D��E����Ԫ��,���ǵĺ˵���������������Ҷ�С��20������C��E�ǽ���Ԫ�أ�A��EԪ��ԭ�ӵ�����㶼ֻ��һ�����ӣ�B��DԪ��ԭ�ӵ�������������ͬ����BԪ��ԭ��L���������K���������3����CԪ��ԭ�ӵ�������������DԪ��ԭ��������������һ����
��1����д��Bԭ�ӵĽṹʾ��ͼ__________���뻭��E���ӵĽṹʾ��ͼ___________
��2���뻭��D���ӵĵ���ʽ___________���뻭��C���ӵĵ���ʽ__________________
��3����д��������B���Ӿ�����ͬ�����������ķ���____________��_____________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ�����ӵ���������ֵ������˵����ȷ����
A. �ں�CO32-����ΪNA��Na2CO3��Һ�У�Na+����Ϊ2NA
B. ��״���£�2.24 L����ͱ�ϩ�Ļ�����к���ԭ����ĿΪ0.6NA
C. ��0.1 mol H2��0.2 mol I2(g)�����ܱ������г�ַ�Ӧ�����ɵ�H-I����ĿΪ0.2NA
D. �����£����1 L�ľ���ʳ��ˮ��һ��ʱ�������ҺpHΪ11�����������ͨ�����ߵĵ�����ĿΪ0.002NA
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com