
| ���� |
| ||
| ||



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����Һ�ʵ����ԣ�������� |
| B����һ�����߷ֱ�ͨ����Һ�ͽ���ʱ������������ԵĹ����ǰ����û�� |
| C����Һ�еķ�ɢ������������ֽ�������з�ɢ�����Ӳ�������ֽ |
| D����FeCl3��Һ�μӵ���ˮ�У���ʱ�������оͿ��Ƶ�Fe��OH��3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
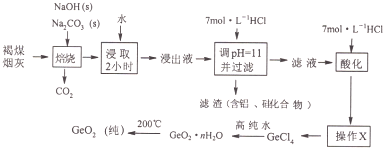
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ij��ɫ��Һ�μ���ˮ��CCl4�������÷ֲ���²���Һ����ɫ����ԭ��Һ����I- |
| B��ȡ������ҺX�������м�������������ˮ���ټӼ���KSCN��Һ����Һ��죬˵��X��Һ��һ������Fe2+ |
| C����ij��Һ�еμ�BaCl2��Һ�����а�ɫ�������ٵμ�����ϡHNO3�����������ܽ⣬��˵��ԭ��Һ��һ����SO42- |
| D��ij��ɫ��Һ�ýྻ��˿պȡ��Һ������ɫ��Ӧ������ʻ�ɫ����ԭ��Һ����Na+��K+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��1 molCO2Ϊ22.4L |
| B����״���£�1molˮ���Ϊ22.4L |
| C����ͬ״���£�1molH2��O2��ռ�����ͬ |
| D��ֻ���ڱ�״��������Ħ���������22.4mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ͼװ����ȡ���е����ָ�����������壨ͼ������̨�����С����ȼ������ռ�װ�þ�����ȥ����Ҫʱ���Լ��ȣ�a��b��c��d��ʾ��Ӧ�����м�����Լ�����������ȷ���ǣ�������
����ͼװ����ȡ���е����ָ�����������壨ͼ������̨�����С����ȼ������ռ�װ�þ�����ȥ����Ҫʱ���Լ��ȣ�a��b��c��d��ʾ��Ӧ�����м�����Լ�����������ȷ���ǣ�������| ѡ�� | ���� | a | b | c | d |
| A | CO2 | ���� | CaCO3 | ����Na2CO3��Һ | Ũ���� |
| B | Cl2 | Ũ���� | MnO2 | NaOH��Һ | Ũ���� |
| C | NH3 | ����NH4Cl��Һ | ��ʯ�� | H2O | ����NaOH |
| D | NO | ϡ���� | ͭм | H2O | Ũ���� |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����Һ����ȡ������ |
| B����ȡ������Һ |
| C����Һ���������� |
| D��������ȡ����Һ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com