
������£�±�����봼��Ӧ�����ѣ��ң��ϣ���������
�ң��أ������ϣ�![]() �ң��ϣ�����+HX
�ң��ϣ�����+HX
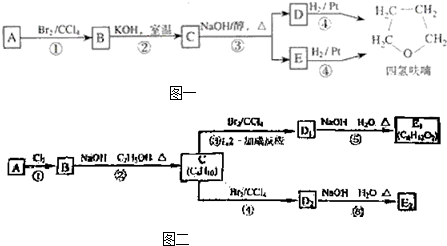
������A�������IJ���Ӧ�ɵõ������ܼ�����ૣ���Ӧ��ͼ���£�

��ش��������⣺
��1��1 molA��1 mol��2��һ��������ǡ�÷�Ӧ�����ɱ���һԪ��Y��Y��̼Ԫ�ص���������ԼΪ65���� ��ٵķ���ʽΪ______ �Ľṹ��ʽΪ____________��
��2���ڢ٢ڲ���Ӧ���ͷֱ�Ϊ ��
��
��3��������B���еĻ�ѧ���ʣ���д��ĸ���ţ���__________________________
a���ɷ���������Ӧ
b��ǿ���ǿ�������¾��ɷ�����ȥ��Ӧ
c���ɷ���������Ӧ
d���������¿ɷ����Ӿ۷�Ӧ
��4��д��D��E�Ľṹ��ʽ��D_________________E_____ ��
��5��д��������C��![]() ˮ��Һ���ȵĻ�ѧ��Ӧ����ʽ������������������
ˮ��Һ���ȵĻ�ѧ��Ӧ����ʽ������������������
��6��д���������״���������ͬ���칹��Ľṹ��ʽ��������������
 �±�Сѧ��Ԫ�Բ���ϵ�д�
�±�Сѧ��Ԫ�Բ���ϵ�д� �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| KOH/���� |




 +NaOH
+NaOH| ˮ |
 +NaBr
+NaBr +NaOH
+NaOH| ˮ |
 +NaBr
+NaBr| �� |
| �� |
| �� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
![]()
������A�������IJ���Ӧ�ɵõ������ܼ�����ૣ���Ӧ��ͼ���£�
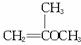
������������⣺
(1)1 mol A��1 mol H2��һ��������ǡ�÷�Ӧ�����ɱ���һԪ��Y��Y��̼Ԫ�ص���������ԼΪ65%����Y�ķ���ʽΪ____________��A���������������ŵ�������____________��A�Ľṹ��ʽΪ____________��
(2)�ڢ٢ڲ���Ӧ���ͷֱ�Ϊ����______________________����______________________��
(3)������B���еĻ�ѧ����(��д��ĸ����)��____________��
a.�ɷ���������Ӧ b.ǿ���ǿ�������¾��ɷ�����ȥ��Ӧ
c.�ɷ���������Ӧ d.�������¿ɷ����Ӿ۷�Ӧ
(4)д��C��D��E�Ľṹ��ʽ��C_______________��D��E_______________��
(5)д��������C��NaOHˮ��Һ��Ӧ�Ļ�ѧ����ʽ��_____________________________��
(6)д���������״���������ͬ���칹��Ľṹ��ʽ��__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
R��X+R��OH![]() R��O��R��+HX
R��O��R��+HX
������A�������IJ���Ӧ�ɵõ������ܼ�����ૣ���Ӧ��ͼ��ͼ1-4-27
ͼ1-4-27
��ش��������⣺
(1)1 mol A��1 mol H2��һ��������ǡ�÷�Ӧ�����ɱ���һԪ��Y��Y��̼Ԫ�ص���������ԼΪ65%����Y�ķ���ʽΪ_____________________________________________��
A���������������ŵ�������____________________________________________��
A�Ľṹ��ʽΪ________________________________________________________��
(2)�ڢ٢ڲ���Ӧ���ͷֱ�Ϊ��__________________��__________________��
(3)������B���еĻ�ѧ����(��д��ĸ����)��_________��
a.�ɷ���������Ӧ
b.ǿ���ǿ�������¾��ɷ�����ȥ��Ӧ
c.�ɷ���������Ӧ
d.�������¿ɷ����Ӿ۷�Ӧ
(4)д��C��D��E�Ľṹ��ʽ��
C__________________��D��E__________________
(5)д��������C��NaOHˮ��Һ��Ӧ�Ļ�ѧ����ʽ��
____________________________________________________________________
(6)д���������״���������ͬ���칹��Ľṹ��ʽ��___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������£�±�����봼��Ӧ������(R��O��R��):
![]()
������A�������IJ���Ӧ�ɵõ������ܼ�����ૣ���Ӧ��ͼ���£�

������������⣺
(1)1 mol A��1 mol H2��һ��������ǡ�÷�Ӧ�����ɱ���һԪ��Y��Y��̼Ԫ�ص���������Ϊ65%����Y�ķ���ʽΪ________________��A���������������ŵ�������_______________��A�Ľṹ��ʽΪ_______________��
(2)�ڢ٢ڲ���Ӧ���ͷֱ�Ϊ��_______________����_______________��
(3)������B���еĻ�ѧ����(��д��ĸ����)��_______________��
a.�ɷ���������Ӧ
b.ǿ���ǿ�������¾��ɷ�����ȥ��Ӧ
c.�ɷ���������Ӧ
d.�������¿ɷ����Ӿ۷�Ӧ
(4)д��C��D��E�Ľṹ��ʽ��
C_____________��D��E______________________________��
(5)д��������C��NaOHˮ��Һ��Ӧ�Ļ�ѧ����ʽ��_______________________________��
(6)д���������״���������ͬ���칹��Ľṹ��ʽ��____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꼪��ʡ������ʮһ�и߶���ѧ���ڳ����Ի�ѧ�Ծ����������� ���ͣ������
��12�֣���֪������£�±�����봼��Ӧ������(R1��O--R2)��
������A�������IJ���Ӧ�ɵõ������ܼ�����ૣ���Ӧ��ͼ����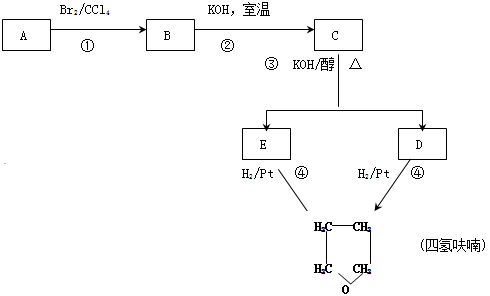
��ش��������⣺
(1)1molA��1molH2��һ��������ǡ�÷�Ӧ�����ɱ���һԪ��Y��Y��̼Ԫ�ص���������ԼΪ65%����Y�ķ���ʽΪ ��A�����������ŵ������� A�Ľṹ��ʽ
(2)�ڢ٢۲���Ӧ�����Ǣ� ��
(3)��֪E�ĺ˴Ź��������г��������,д��C����E�Ļ�ѧ����ʽ
(4)A�кܶ��칹��,�����ܺ�������ͭ��Ӧ���ɺ�ɫ�������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com