
���� ��2������װ����ʽ�ζ����У�
��3��NaOH��Һ�ü�ʽ�ζ�����ȡ��ת�Ƶ���ƿ�У�ѡ���̪��ָʾ������ҺΪ��ɫ���ζ��յ�ʱ��Ϊ��ɫ��
��4�����HCl+NaOH=NaCl+H2O��n��HCl��=0.02L��0.2mol/L=0.004mol���㣻
��5������������NaOH��HCl�����ʵ�����ƫСʱ��ʵ������С��82%��
��� �⣺��2����������װ��25.00mL����ʽ�ζ����У�����Һ��λ���ڡ�0���̶����£�����¼�¿̶ȣ�
�ʴ�Ϊ����ʽ��
��3��ȡ20.00mL����Һ������ʵ�����ʹ�õ���Ҫ�����м�ʽ�ζ��ܺ���ƿ���÷�̪��ָʾ��ʱ���ζ�����Һ��ɫ�ɺ�ɫ�պñ����ɫΪֹ��
�ʴ�Ϊ����ʽ�ζ��ܣ���ƿ���죻�ޣ�
��4���ζ����յ����������ȥ20.00mL��n��HCl��=0.02L��0.2mol/L=0.004mol����HCl+NaOH=NaCl+H2O����֪n��NaOH��=0.004mol�����������Ƶ���������Ϊ$\frac{0.004mol��40g/mol��\frac{500}{20}}{5.0g}$��100%=80%��
�ʴ�Ϊ��80%��
��5��A��ת�ƴ���Һ������ƿʱ��δϴ���ձ���NaOH�����ʵ���ƫС��ʵ����ƫС����Aѡ��
B����ʽ�ζ���������ˮϴ�Ӻ�ֱ��װ���ᣬ�����Ũ��ƫС����������ƫ��ʵ����ƫ��B��ѡ��
C���ζ�ʱ��Ӧ��ҡ��̫���ң�������Һ�彦����NaOH�����ʵ���ƫС��ʵ����ƫС����Cѡ��
D���ζ����յ�ʱ���ζ��ܼ����������ݣ���������ƫ��ʵ����ƫ��D��ѡ��
E���ζ���ʼʱ�������ӣ��յ�ʱ�������ӣ���������ƫС��ʵ����ƫС����Eѡ��
�ʴ�Ϊ��ACE��
���� ���⿼�����ʺ����IJⶨʵ�飬Ϊ��Ƶ���㣬����������ʹ�á��кͷ�Ӧ��ʵ�鼼��Ϊ���ؼ������ط�����ʵ�������Ŀ��飬ע���Ϸ�Ӧ���㣬��Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaHSO4��Һ�м���Ba��OH��2��Һ��ǡ��������Ba2++OH-+H++SO42-�TBaSO4+H2O | |
| B�� | �����ʯ��ˮ��ͨ�����CO2��OH-+CO2�THCO3- | |
| C�� | ����������Һ��ϡ H2SO4 ��Ӧ��Ba2++SO42-�TBaSO4�� | |
| D�� | ̼��������ᷴӦ��CO32-+2H+�TH2O+CO2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������Ԫ�صĻ�̬ԭ���У�CrԪ�ص�δ�ɶԵ�������� | |
| B�� | ��������Ԫ����3pԭ�ӹ����һ��δ�ɶԵ��ӵ�ԭ����2�� | |
| C�� | ���������Ϊż���Ļ�̬ԭ�ӣ���ԭ�ӹ���п��ܺ��С�δ�ɶԵ��ӡ� | |
| D�� | ��̬̼ԭ����δ�ɶԵ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ˮ����ȡ�壬���þƾ�����ȡ�� | |
| B�� | ����ʵ����Ϻ���������ը�ѣ�������Ϊû�е�ʯ���� | |
| C�� | ��ȡ��ˮ��Һ�е⣬��������Ȼ�̼��Һʱ���۾�ע�ӷ�Һ©����Һ�� | |
| D�� | ����ʱ��Ϊ�˼ӿ�ˮ�����٣�ˮӦ���Ͽڽ��룬�¿����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��� | ʵ������ | ʵ������ | ���ӷ���ʽ | ʵ����� |
| �� | ��FeCl2��Һ�е���������ˮ | ��Һ��dz��ɫ��Ϊ�ػ�ɫ | Fe2+���л�ԭ�� | |
| �� | ��FeCl2��Һ�м���пƬ | ����д�� | Zn+Fe2+�TZn2++Fe | |
| �� | ��FeCl3��Һ�м����������� | Fe+2Fe3+�T3Fe2+ | Fe3+���������� | |
| �� | ����� | Fe3+���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��ѧ�� | C=O��CO2�� | C=O��COS�� | C=S | H-S | H-O |
| E/��KJ•mol-1�� | 803 | 742 | 577 | 339 | 465 |
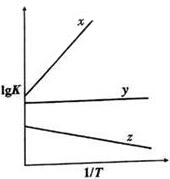
| �ﵽƽ�������ʱ��/min | a����ֵ | b����ֵ | |
| ����A | t | a1 | b1 |
| ����B | 2t | a2 | b2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
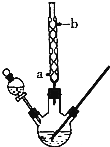 ij��ѧ����С���������ͼ��ʾ��װ����ȡ����������ͼ�мг������ͼ���װ������ȥ������ش��������⣺
ij��ѧ����С���������ͼ��ʾ��װ����ȡ����������ͼ�мг������ͼ���װ������ȥ������ش��������⣺| �Ҵ� | ���� | �������� | 98%Ũ���� | |
| �۵�/�� | -117.3 | 16.6 | -83.6 | - |
| �е�/�� | 78.5 | 117.9 | 77.5 | 338.0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
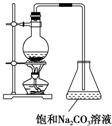 ������Ҫ���л��ϳ��м��壬�㷺Ӧ�����ܼ������ܼ������ϡ��ϼ���ӡˢ����֯�ȹ�ҵ������������ʵ���Һ�ҵ�Ʒ����������·�Ӧ��CH3COOH+C2H5OH$?_{��}^{ŨH_{2}SO_{4}}$CH3COOC2H5+H2O�������Ҫ��ش��������⣺
������Ҫ���л��ϳ��м��壬�㷺Ӧ�����ܼ������ܼ������ϡ��ϼ���ӡˢ����֯�ȹ�ҵ������������ʵ���Һ�ҵ�Ʒ����������·�Ӧ��CH3COOH+C2H5OH$?_{��}^{ŨH_{2}SO_{4}}$CH3COOC2H5+H2O�������Ҫ��ش��������⣺| ͬһ��Ӧʱ�� | ͬһ��Ӧ�¶� | |||||
| ��Ӧ�¶�/�� | ת���ʣ�%�� | ѡ���ԣ�%��* | ��Ӧʱ��/h | ת���ʣ�%�� | ѡ���ԣ�%��* | |
| 40 | 77.8 | 100 | 2 | 80.2 | 100 | |
| 60 | 92.3 | 100 | 3 | 87.8 | 100 | |
| 80 | 92.6 | 100 | 4 | 92.3 | 100 | |
| 120 | 94.5 | 98.7 | 6 | 93.0 | 100 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �Ȼ��ơ�ʳ�Ρ�NaCl2 | B�� | ̼������ ���NaHCO3 | ||
| C�� | �������ơ��ռNaOH | D�� | �������ơ���ʯ�ҡ�CaO |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com