

 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д� ��˼άС�ھ�100����ҵ��ϵ�д�
��˼άС�ھ�100����ҵ��ϵ�д� ��ʦָ��һ��ͨϵ�д�
��ʦָ��һ��ͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2011?����һģ�����ͷ����ǻ���ԭ�ϣ����ڷ������ʻ��ã���������ȡ������
��2011?����һģ�����ͷ����ǻ���ԭ�ϣ����ڷ������ʻ��ã���������ȡ�������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ͷ����ǻ���ԭ�ϡ����ڷ������ʻ��ã���������ȡ������
��1������ͨ����ⴿ����Һ̬HF���F2����ԭ����_________�������Һ̬KHF2ʱ�������ֱ�õ�F2��H2��д��������HF2���ŵ��������ĵ缫��Ӧʽ______________����2�����û�ѧ��ӦҲ����ȡ��������ƽ���л�ѧ����ʽ��
____K2MnF6+____SbF5��____KSbF6+____MnF3+____F2�������л�ԭ������________��
��3��ij�¶��²��Ũ�Ⱦ�Ϊ0.10mol/L��KF��HF���Һ��pH=4�����Ƽ���HF�ĵ���ƽ�ⳣ��Ka(д���������)��
��4��һ��Ũ�ȵ�HF��Al2(SO4)3���Һ�У����ĸ���������f��PH�ķֲ�������ͼʾ����NaOHʹ���Һ��pH��5������7��д���йط�Ӧ�����ӷ���ʽ��_____________________________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ͷ����ǻ���ԭ�ϡ����ڷ������ʻ��ã���������ȡ������
��1������ͨ����ⴿ����Һ̬HF���F2����ԭ����_________�������Һ̬KHF2ʱ�������ֱ�õ�F2��H2��д��������HF2���ŵ��������ĵ缫��Ӧʽ______________����2�����û�ѧ��ӦҲ����ȡ��������ƽ���л�ѧ����ʽ��
____K2MnF6+____SbF5��____KSbF6+____MnF3+____F2�������л�ԭ������________��
��3��ij�¶��²��Ũ�Ⱦ�Ϊ0.10mol/L��KF��HF���Һ��pH=4�����Ƽ���HF�ĵ���ƽ�ⳣ��Ka(д���������)��
��4��һ��Ũ�ȵ�HF��Al2(SO4)3���Һ�У����ĸ���������f��PH�ķֲ�������ͼʾ����NaOHʹ���Һ��pH��5������7��д���йط�Ӧ�����ӷ���ʽ��_____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ������ѧ������ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
(14��)���ͷ����ǻ���ԭ�ϡ����ڷ������ʻ��ã���������ȡ������
��1������ͨ����ⴿ����Һ̬HF���F2����ԭ���� �������Һ̬KHF2ʱ�������ֱ�õ�F2��H2��д��������HF2- �ŵ��������ĵ缫��Ӧʽ ��
��2������K2MnF6��SbF5��һ�������·�����ӦҲ����ȡ����ͬʱ����KSbF6��MnF3����ѧ����ʽΪ �����л�ԭ������ ��
��3����֪�����������д�������ƽ�⣺H3F3 3HF ��H��0, H2F2
3HF ��H��0, H2F2 2HF ��H��0��
2HF ��H��0��
�����ڶ��¶����������ٳ���H3F3����H3F3��HF��Ũ�ȣ�mol��L����ֵ ���������С�����䡱����ͬ����
�����ڶ��¶����������ٳ���HF����H3F3��HF ��Ũ�ȣ�mol��L����ֵ ��
��4��һ��Ũ�ȵ�HF��Al2(SO4)3���Һ�У����ĸ���������?��pH�ķֲ�������ͼʾ����NaOHʹ���Һ��pH��5������7��д���йط�Ӧ�����ӷ���ʽΪ
��
_
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ������ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
(14��)���ͷ����ǻ���ԭ�ϡ����ڷ������ʻ��ã���������ȡ������
��1������ͨ����ⴿ����Һ̬HF���F2����ԭ���� �������Һ̬KHF2ʱ�������ֱ�õ�F2��H2��д��������HF2- �ŵ��������ĵ缫��Ӧʽ ��
��2������K2MnF6��SbF5��һ�������·�����ӦҲ����ȡ����ͬʱ����KSbF6 ��MnF3����ѧ����ʽΪ �����л�ԭ������ ��
��3����֪�����������д�������ƽ�� ��H3F3 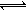 3HF ��H ��0, H2F2
3HF ��H ��0, H2F2 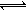 2HF ��H ��0��
2HF ��H ��0��
�����ڶ��¶����������ٳ���H3F3����H3F3��HF��Ũ�ȣ�mol��L����ֵ ���������С�����䡱����ͬ����
�����ڶ��¶����������ٳ���HF����H3F3��HF ��Ũ�ȣ�mol��L����ֵ ��
��4��һ��Ũ�ȵ�HF��Al2(SO4)3���Һ�У����� ����������ƒ��pH�ķֲ�������ͼʾ����NaOHʹ���Һ��pH��5������7��д���йط�Ӧ�����ӷ���ʽΪ
��

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com