
ijҺ̬±����RX(R�������X��ij��±��ԭ��)���ܶ���a g/cm3����RX���Ը�ϡ���ˮ�ⷴӦ����ROH(�ܸ�ˮ����)��HX��Ϊ�˲ⶨRX����Է����������ⶨ��ʵ�鲽�����£�
��ȷ��ȡ��±����b mL��������ƿ�С�
������ƿ�м������ϡNaOH��Һ�����ϴ��в����ܵ����ӣ����ȣ�������Ӧ��
�۷�Ӧ��ɺ���ȴ��Һ����ϡHNO3�ữ���μӹ���AgNO3��Һ�õ���ɫ������
�ܹ��ˡ�ϴ�ӣ��������أ��õ�����c g��
�ش��������⣺
(1)װ���в����ܵ�������________��
(2)������У�ϴ�ӵ�Ŀ����Ϊ�˳�ȥ������������________���ӡ�
(3)��±����������±�ص�������_______���жϵ�������______��
(4)��±��������Է���������________(�г���ʽ)��
(5)����ڲ�����У�����HNO3�������㣬û�н���Һ�ữ��������в�õ�cֵ________(������ѡ�����)��
A��ƫ�� B��ƫС
C������ D��������
��������ʵ��ķ�Ӧԭ���ǣ�
R��X��NaOH�D��R��OH��NaX��
NaX��AgNO3===AgX����NaNO3��
(1)��R��X���ۡ��е�ϵͣ�����ʱ�ӷ�������װ���еij������ܵ������Ƿ�ֹ±�����ӷ�(�����������)��
(2)R��OH��Ȼ����ˮ���ܣ������Ե��룬���Գ���AgX������������ֻ����Na����NO ������Ag����
������Ag����
(3)������±����AgX�����ǰ�ɫ�ģ����Ը�±������������±�����ȡ�
(4)R��Cl����������������������AgCl
M������������������������ 143.5
a g��cm��3��b mL���������������� c g
 ��
��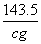
M�� ��
��
(5)���������㣬��������õĹ��廹�����Ag2O��ʹ������cƫ��
�𰸣�(1)��ֹ±�����ӷ�(�����������)
(2)Ag����Na����NO
(3)�ȡ��õ���±���������ǰ�ɫ��
(4) ��(5)A
��(5)A


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��Ԫ�����ڱ������ڵ�һ���֣�A��B����Ԫ�ؿ����γɹ��ۻ�����B2A2������������ԭ��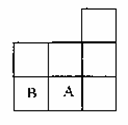 ���������ﵽ8�����ӵ��ȶ��ṹ������˵������ȷ���ǣ� ��
���������ﵽ8�����ӵ��ȶ��ṹ������˵������ȷ���ǣ� ��
A���û�����������״̬���ܵ���
B���û�����Ľṹʽ��Cl-S-S-Cl
C�û������γɵľ����Ƿ��Ӿ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������������գ���Ŀǰ����ˮ���塱������Ҫ����֮һ���乤���������£�
 |
��1������ܵ����ӷ�Ӧ����ʽΪ ��
��2��������ˮ���������������������õ�Һ������ˮ�Ļ������ǵ�����ܶ����ϴ����Һ���ʵ���ҷ���Ϊ ��
��3������������ʵ�����н��У����õ��IJ��������� ��
��4����ҵ������ֱ��������ĺ�ˮ�õ�Һ�壬��Ҫ����������������SO2���ա��Ȼ�����ԭ���� ��
��5����±ˮ������������ȡ����þ���û�ѧ����ʽ��ʾ�ӿ�±ˮ��ȡ����þ�ķ�Ӧԭ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪����RNH2��R��CH2Cl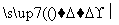 RNHCH2R�䣫HCl(R��R���������)
RNHCH2R�䣫HCl(R��R���������)
�ڱ���ͬϵ���ܱ���������������磺
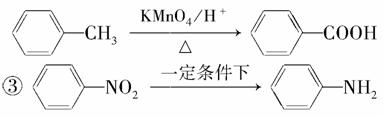
������������ ��(�����������ԣ��ױ�����)
������C����ȡ������(����ʹ)���м�����ϳ�·����ͼ��ʾ��
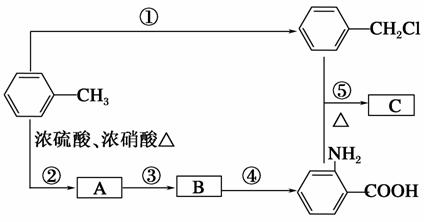
��ش��������⣺
(1)B���ʵĽṹ��ʽ��______________________��
(2)д����Ӧ�١��ڵĻ�ѧ����ʽ��
��____________________________________________________��
��____________________________________________________��
(3)��Ӧ�١����У�����ȡ����Ӧ����________(�Ӧ���)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij±������C5H11Cl������ȥ��Ӧʱ�����Եõ�����ϩ�������±�������Ľṹ��ʽ����Ϊ(����)
A��CH3CH2CH2CH2Cl
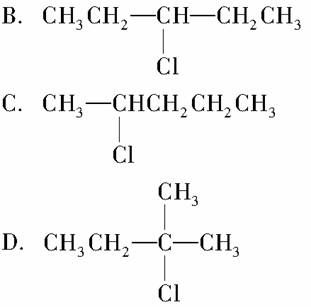
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NaOH��������1. 0 mol/L��NaOH��Һ220 mL������˵����ȷ����
0 mol/L��NaOH��Һ220 mL������˵����ȷ����
(����)
A�����ȳ�ȡNaOH����10g
B������ʱ���ӿ̶���ʹ�����Ƶ���ҺŨ��ƫ��
C�����ݺ���Һ���ȣ�����ʱ����Һ����ڿ̶��ߣ������ּ�����ˮ���̶���
D������ƿ��ԭ����������ˮ�Խ��û��Ӱ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һƿ14%��KOH��Һ������������100 g ˮ��Ϊ28%��KOH��Һ80 mL����80 mL ��Һ�����ʵ���Ũ��Ϊ(����)
A��5 mol/L B��6 mol/L
C��6.25 mol/L D��6.75 mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
þ���������������Dz����ٵ�ԭ��,��ҵ����þ�۵����һ���ǽ�þ��������������ȴ,���п���Ϊ��ȴ������ǣ� ��
A������
B��
C��
D��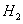
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ�������(����)
A����Ba(OH)2��Һ�еμ�ϡ���
Ba2����2OH����2H����SO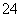 ===BaSO4����2H2O
===BaSO4����2H2O
B�����Խ�����KMnO4����H2O2��
2MnO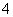 ��5H2O2��6H��===2Mn2����5O2����8H2O
��5H2O2��6H��===2Mn2����5O2����8H2O
C�������ʵ�����MgCl2��Ba(OH)2��HCl��Һ��ϣ�Mg2����2OH��===Mg(OH)2��
D��Ǧ�����س��ʱ��������Ӧ��PbSO4��2H2O��2e��===PbO2��4H����SO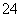
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com