
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��101��Уͬ����ϰ����һ��ѧ���˽���(�¿α�B��2004�����ͨ��) �˽�ʵ��� ���ͣ�022
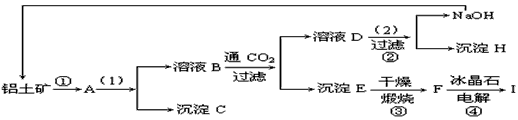
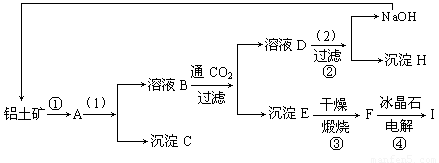
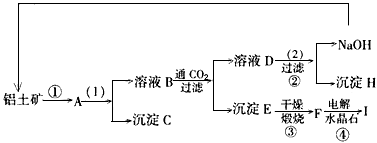
��ҵ�Ʊ���һ���Ǵ�������(��Ҫ�ɷ���Al2O3������Fe2O3����)�еõ���������Al2O3��Ȼ������Al2O3�õ�������ͼ�Ǵ����������ᴿ��Al2O3�ļ�ʾ��ͼ������ǣ�浽��һ����Ӧ�ǣ�2NaAlO2��CO2��3H2O��Na2CO3��2Al(OH)3��
(1)д��ͼʾ��(1)��ʵ�������________��ͼʾ��(2)������Լ�________��
(2)���ƶ�����(д��ѧʽ)B________��C________��H________��F________��
(3)д����ѧ����ʽ��
��________________����________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���ս̰���л�ѧ����2 4.1��ѧ����ʶ�ʹ������ʵĿ�ѧ��ϰ���������棩 ���ͣ������
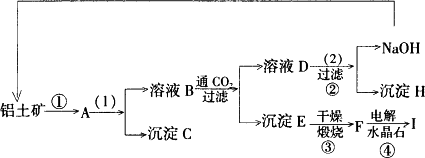
��ҵ�Ʊ���һ���Ǵ���������Ҫ�ɷ���Al2O3������Fe2O3���ʣ��еõ���������Al2O3��Ȼ������Al2O3�õ�������ͼ�Ǵ����������ᴿ��Al2O3�ļ�ʾ��ͼ������ǣ�浽��һ����Ӧ�ǣ�2NaAlO2��CO2��3H2O��Na2CO3��2Al(OH)3����
��1��д��ͼʾ�У�1����ʵ������� ��ͼʾ�У�2��������Լ� ��
��2�����ƶ����ʣ�д��ѧʽ��B ��C ��H ��F ��
��3��д����ѧ����ʽ��
�� ��
�� ��
�� ��
�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ�ʵ����


 CO����H2O ��������¸�װ�ý���ѡ�����һ����Խ�Ϊ������ʵ��װ��ͼ(ijЩװ�ÿ��ظ�ʹ��)
CO����H2O ��������¸�װ�ý���ѡ�����һ����Խ�Ϊ������ʵ��װ��ͼ(ijЩװ�ÿ��ظ�ʹ��) 
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����ĩ�� ���ͣ������
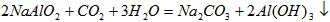
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com