
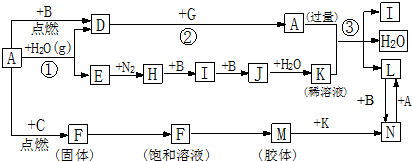
���� B��C��E��I��JΪ���壬����CΪ����ɫ���壬��C��Cl2��JΪ����ɫ���壬��J��NO2��DΪ��ɫ���壬MΪ���ɫ���壬MΪFe��OH��3��A��GΪ�ճ������еij���������A�ܺ�ˮ������Ӧ����A��Fe������B��Ӧ����D��B�����壬��D��Fe3O4��BΪO2��E��H2��F��FeCl3��H��NH3��IΪNO��JΪNO2��KΪHNO3��G�������г����������ܷ����û���Ӧ����G��Al����������ϡ���ᷴӦ����L��L��Fe��NO3��2��������������������N��NΪFe��NO3��3�������Ŀ�������
��� �⣺B��C��E��I��JΪ���壬����CΪ����ɫ���壬��C��Cl2��JΪ����ɫ���壬��J��NO2��DΪ��ɫ���壬MΪ���ɫ���壬MΪFe��OH��3��A��GΪ�ճ������еij���������A�ܺ�ˮ������Ӧ����A��Fe������B��Ӧ����D��B�����壬��D��Fe3O4��BΪO2��E��H2��F��FeCl3��H��NH3��IΪNO��JΪNO2��KΪHNO3��G�������г����������ܷ����û���Ӧ����G��Al����������ϡ���ᷴӦ����L��L��Fe��NO3��2��������������������N��NΪFe��NO3��3��
��1��AΪFe��AԪ��λ�ڵ������ڵ�VIII�壻D�Ļ�ѧʽΪFe3O4��K�Ļ�ѧʽΪHNO3��
�ʴ�Ϊ�����ģ�VIII��Fe3O4��HNO3��
��2����20mL��ˮ�еμ�F������Һ1mL��2mL��������У�����Һ�ʺ��ɫ�����Ƶ�M���壬������ˮ�����������������壬������е����ӷ���ʽFe3++3H2O?Fe��OH��3�����壩+3H+��
�ʴ�Ϊ��Fe3++3H2O?Fe��OH��3�����壩+3H+��
��3��L��Fe��NO3��2��NΪFe��NO3��3������������dz��ɫ���������Ի�ɫ�����Լ���Fe��NO3��2��Һ��Fe��NO3��3��Һ��������������ǹ۲���Һ����ɫ��
�ʴ�Ϊ���۲���Һ����ɫ��
��4����������ϡ���ᷴӦ��������������һ��������ˮ�����ӷ���ʽΪ3Fe+2NO3-+8H+�T3Fe2++2NO��+4H2O��
�ʴ�Ϊ��3Fe+2NO3-+8H+�T3Fe2++2NO��+4H2O��
���� ���⿼������������������Ʊ������ȷ�Ӧ��Ԫ��λ�õ��жϵ�֪ʶ�㣬��ȷ���������������ǽⱾ��ؼ�����C��J��MΪͻ�ƿڣ������������ϵķ��������ƶϣ�ע�⣺�������������Ʊ������в����ò��������裬���ܳ�ʱ����к��ɫҺ�壬��������������Ϊ�״��㣮


 ������ȫ�̼����ĩ���100��ϵ�д�
������ȫ�̼����ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڢۢߢ��� | B�� | �٢ڢۢݢ�� | C�� | �ڢݢޢߢ� | D�� | �ڢܢߢ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
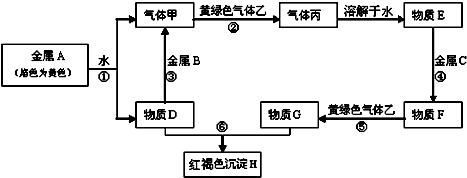
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ø����pH��ֽ�ⶨNaClO��Һ��pH | |
| B�� | �����µ�ʯӢ�����н����ۻ��������ƹ����ʵ�� | |
| C�� | �÷�Һ©�������屽��ˮ�Ļ����ʱ���屽���¿ڷų���ˮ���Ͽڵ��� | |
| D�� | ��������������Ϊ10%������ͭ��Һ����ȷ��ȡ10 g����ͭ��������90 gˮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | �ڢڲ���Ӧ�Ļ�ѧ����ʽΪ��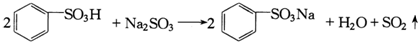 | |
| B�� | �ڢ۲���Ӧ�г��˱����ƻ�������Na2SO4 | |
| C�� | �ڢܲ���Ӧ�ɱ�ʾΪ��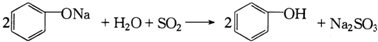 | |
| D�� | ����������Ӧ�ж�����ǿ��Ϊ��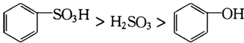 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ư�ۺ�����������������ˮ�Ĵ��������ߵ�����ԭ������ͬ�� | |
| B�� | Na2SiO3�Dz�����ˮ���Σ������轺 | |
| C�� | �����������������IJ��� | |
| D�� | ʵ�����ô����������Լ�ƿ��������������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ȥCO2�����е�HCl��ͨ�������ı���̼������Һ | |
| B�� | ��ȥNaCl��������MgCl2���ܽ���������NaOH��Һ�����˺�����Һ�м����������ᣬ�����ᾧ | |
| C�� | ��ȥFeCl2��Һ�е�FeCl3������������ͭ�ۣ����� | |
| D�� | ��ȥAl2O3�е�����SiO2������������NaOH��Һ������ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com