
ijѧϰС��Ϊ�ˡ��ⶨ��þ�Ͻ�������������������ȡ8.0g��������þ�Ͻ���Ʒ�������3��ʵ�鷽�����£�k*s5u**
��������þ�Ͻ� ���������������ΪVmL(��״��)
![]()
�ҷ�������þ�Ͻ� ���ʣ����������Ϊ2.4g
![]()
![]()
����������þ�Ͻ� ��Һ ��ó���������Ϊag
��1�������ܷ�ⶨ������������������˵������ ��
��2���ҷ����з�Ӧ�����ӷ���ʽΪ �����������������Ϊ ��
��3���������мӹ�����NaOH��Һ��ַ�Ӧ���ˡ�ϴ�ӡ���ɳ����������ʱ���¶�ƫ�ߣ�������һ���ַֽ⣬����������������� �����ƫ����ƫС������Ӱ�족��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
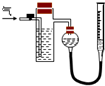 ��2010?��̨һģ�����Ṥ����Χ�Ŀ������н϶�Ķ�������ij�о���ѧϰС��Ϊ�˲ⶨ����
��2010?��̨һģ�����Ṥ����Χ�Ŀ������н϶�Ķ�������ij�о���ѧϰС��Ϊ�˲ⶨ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ���������һ����Ҫ�Ļ�ѧ�Լ�������Һ�����ȶ��������������»�ֽ����ɶ������̺������������Ի���������Һ�зֽ��ٶȺ���������ֽ��ٶȼӿ죮
���������һ����Ҫ�Ļ�ѧ�Լ�������Һ�����ȶ��������������»�ֽ����ɶ������̺������������Ի���������Һ�зֽ��ٶȺ���������ֽ��ٶȼӿ죮| ������������Һ�Ĵ���ÿ����Һ�������ͬ�� | ���������Һ��ɫ��ȥ��ʱ�� |
| �ȵ����1�� | 1min |
| ��ɫ���ٵ����2�� | 15s |
| ��ɫ���ٵ����3�� | 3s |
| ��ɫ���ٵ����4�� | 1s |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������᳧��Χ�Ŀ����к��н϶�SO2��ijѧϰС��Ϊ�˲ⶨ������SO2�����������������ʵ�飺
ȡ������״���µĿ���1 L���ں�N2��O2��SO2��CO2��������ͨ��������ˮ��Ȼ���ڴ���Һ�м��������BaCl2��Һ��������ɫ���������˺���ϴ������������Ϊ0.233 g![]() ��ش�
��ش�
��1��ͨ��������ˮ��Ŀ���ǣ� ��
д����ط�Ӧ�����ӷ���ʽ�� ��
��2�����������BaCl2��Һ��Ŀ���ǣ� ��
д����ط�Ӧ�����ӷ���ʽ�� ��
��3�����˺�������Һ�м�K2SO4��Һ���а�ɫ����������֤���� ��
��4��������ˮϴ�ӳ���2--3�Σ�ϴ�ӳ�����ʵ������ǣ�
��
����ϴҺ�м���AgNO3��Һ���������֣�֤���� ��
��5�� �Լ��������SO2���������Ϊ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��16�֣��������᳧��Χ�Ŀ����к��н϶�SO2��ijѧϰС��Ϊ�˲ⶨ������SO2�����������������ʵ�飺
ȡ������״���µĿ���1 L���ں�N2��O2��SO2��CO2��������ͨ��������ˮ��Ȼ���ڴ���Һ�м��������BaCl2��Һ��������ɫ���������˺���ϴ������������Ϊ0.233 g����ش�
��1��ͨ��������ˮ��Ŀ���ǣ� ��
д����ط�Ӧ�����ӷ���ʽ�� ��
��2�����������BaCl2��Һ��Ŀ���ǣ� ��
д����ط�Ӧ�����ӷ���ʽ�� ��
��3�����˺�������Һ�м�K2SO4��Һ���а�ɫ����������֤���� ��
��4��������ˮϴ�ӳ���2--3�Σ�ϴ�ӳ�����ʵ������ǣ�
��
����ϴҺ�м���AgNO3��Һ���������֣�֤���� ��
��5�� �Լ��������SO2���������Ϊ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com