
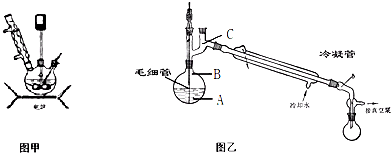
(10��)Ϊ�ⶨ�Ȼ��غ�����ػ�������Ȼ��صĺ�����ijͬѧ���������ʵ�飺������Ʒ���ܽ⣬��������A��Һ�����ˣ��ó�������ҺB��������ϴ�ӣ���ɣ�������C��
�ش��������⣺
��1����Һ�����ʵĻ�ѧʽ_____������C�Ļ�ѧʽ____��
��2����������ƽ������Ʒ������ƽƽ��ʱ���������̴�����������Ϊ26g������Ϊ0.4g����������Ʒʵ��������____��
��3��������ʹ�õ���������Ʒ���У���ֽ������̨����Ȧ���ձ���©��������Ҫ�������������Ʒ��______��
��4����C������Ϊ23.3g����ԭ��������Ȼ��ص���������Ϊ______��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ˮ������Ϊһ����Ҫ�ľ�ϸ����ԭ�ϣ���ũҩ��ҽҩ���л��ϳ����й㷺��;�� �����ط��Ʊ�ˮ���£��ɷ�Ϊ�����Σ���һ��Ϊ�����Ȼ��Σ��ڶ���Ϊ����ˮ��Σ��ܷ�Ӧ����ʽΪ����NH2��2CO+NaClO+2NaOH��H2N-NH2?H2O+NaCl+Na2CO3��
ˮ������Ϊһ����Ҫ�ľ�ϸ����ԭ�ϣ���ũҩ��ҽҩ���л��ϳ����й㷺��;�� �����ط��Ʊ�ˮ���£��ɷ�Ϊ�����Σ���һ��Ϊ�����Ȼ��Σ��ڶ���Ϊ����ˮ��Σ��ܷ�Ӧ����ʽΪ����NH2��2CO+NaClO+2NaOH��H2N-NH2?H2O+NaCl+Na2CO3���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
(10��).������������ʡ������Ⱦ��Ϊ���أ����������������ü�����⡣
�����������һ�������ȼҵ��Ʒ������������������ķ������������£� ( I ������SO2�ķ���ͨ���ⱥ��ʳ��ˮ������Һ�У���NaHSO3��Һ��������ⱥ��ʳ��ˮ�������巴Ӧ���Ƶ����ᡣ��III�����������NaHSO3��Һ�е�SO2������գ����ɵ�NaClѭ�����á�
�� д�����裨I����Ӧ�Ļ�ѧ����ʽ��
�� д�����裨III����Ӧ�����ӷ���ʽ��
�ƻ���ѧ��������� Fe 2ʮ��Fe3ʮ�����ӵĴ����ã������½�SO2������SO42һ��ʵ��SO2�Ļ������á�ij�о���ѧϰС��ݴ���������·�������ʵ���������²ⶨת������SO2������SO42һ��ת���ʡ�
�� ��С�������ͼװ����ʵ���Ҳⶨģ��������SO2�����������X��Һ�����ǣ�����ţ� �����ο�����ԭ��SO2 > I- >Br->Cl-��
A����ĵ�����Һ B�����Ը��������Һ
C������������Һ D���Ȼ�����Һ
�� ������ʵ�����ڱ�״���½��еģ�X����֪��������ʵ���Ũ�ȵ���Һ�����ⶨת������SO2������SO42һ��ת���ʣ���֪�������٣�����ⶨ�������� �ͼ��������ữ���Ȼ�����Һ�����ɳ�����������
��3��Ϊ��һ������SO2����Ⱦ�����Ϊ������������̽����CO��ԭSO2�õ�������ķ�������ȥSO2���÷����漰���Ļ�ѧ��ӦΪ��SO2��2CO��2CO2��Sx����
CO��Sx��COS��2COS��SO2��2CO2��
Sx��������COS�С�C�����ϼ�Ϊ ��
��4����������ˮ�к������ĵ������ͨ��������Ĥ�ѵ����ս��д���������������ϸ���������½�NH4������ΪNO3����NH4����2O2��NO3����2H����H2O��Ȼ�����״�(CH3OH)��NO3���ͼ״�ת��Ϊ���������塣��д������״���Ӧ�����ӷ���ʽ ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ��ɫ��У������һ��������ѧ�Ծ� ���ͣ�ʵ����
(10��)8��34gFeS04��7H20��Ʒ������ˮ���̵���������(��Ʒ�������¶ȱ仯������)����ͼ��ʾ��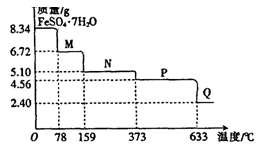
��ش��������⣺
(1)��ȷ��78��ʱ��������M�Ļ�ѧʽ�� ��
(2)ȡ����380��ʱ���õ���ƷP����������������650�棬�õ�һ�ֹ�������Q��ͬʱ��������ɫ�������ɣ�д���÷�Ӧ�Ļ�ѧ����ʽ
(3)ij��ȤС������ͼ��ʾװ�����ʵ�飬��֤(2)�����ɵ���̬���ʣ����ⶨ�ѷֽ��P������(������װ���ڿ�����Ӱ��)��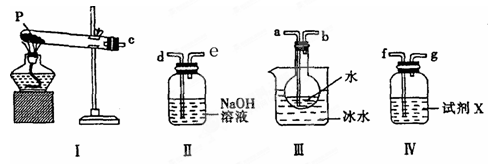
���Լ�X�������� ��
�ڰ������������Ӹ�����������ĸ��ʾ�ӿڵ�����˳��c�� ��
�۳�ַ�Ӧ������װ��III��Բ����ƿ�ڻ����ⶨ�ѷֽ��P�����������������Ϊ����һ������Բ����ƿ����μ����Ȼ�����Һ��ֱ��������ȫ���ڶ��������˻����ڹ������Ͻ�����ϴ����ɲ���ȴ�����£����ء���������������ɡ���ȴ������ֱ���������γ��������������0��1gΪֹ�������յõ�����������ΪWg�����ѷֽ��P������ (�����ʽ) ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ��ɫ��У������һ��������ѧ�Ծ� ���ͣ�ʵ����
(10��)8��34gFeS04��7H20��Ʒ������ˮ���̵���������(��Ʒ�������¶ȱ仯������)����ͼ��ʾ��

��ش��������⣺
(1)��ȷ��78��ʱ��������M�Ļ�ѧʽ�� ��
(2)ȡ����380��ʱ���õ���ƷP����������������650�棬�õ�һ�ֹ�������Q��ͬʱ��������ɫ�������ɣ�д���÷�Ӧ�Ļ�ѧ����ʽ
(3)ij��ȤС������ͼ��ʾװ�����ʵ�飬��֤(2)�����ɵ���̬���ʣ����ⶨ�ѷֽ��P������(������װ���ڿ�����Ӱ��)��

���Լ�X�������� ��
�ڰ������������Ӹ�����������ĸ��ʾ�ӿڵ�����˳��c�� ��
�۳�ַ�Ӧ������װ��III��Բ����ƿ�ڻ����ⶨ�ѷֽ��P�����������������Ϊ����һ������Բ����ƿ����μ����Ȼ�����Һ��ֱ��������ȫ���ڶ��������˻����ڹ������Ͻ�����ϴ����ɲ���ȴ�����£����ء���������������ɡ���ȴ������ֱ���������γ��������������0��1gΪֹ�������յõ�����������ΪWg�����ѷֽ��P������ (�����ʽ) ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com