
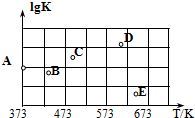
 £¬ŅŅ±½ĶŃĒā·“Ó¦µÄĘ½ŗā³£ŹżKČē±ķĖłŹ¾£»ŌŚĢå»żĪŖ1 LµÄŗćČŻĆܱÕČŻĘ÷ÖŠ³äČė0.2 molŅŅ±½ÕōĘū£¬ŌŚ²»Ķ¬ĪĀ¶ČĻĀ½ųŠŠŅŅ±½ĶŃĒā·“Ó¦£®
£¬ŅŅ±½ĶŃĒā·“Ó¦µÄĘ½ŗā³£ŹżKČē±ķĖłŹ¾£»ŌŚĢå»żĪŖ1 LµÄŗćČŻĆܱÕČŻĘ÷ÖŠ³äČė0.2 molŅŅ±½ÕōĘū£¬ŌŚ²»Ķ¬ĪĀ¶ČĻĀ½ųŠŠŅŅ±½ĶŃĒā·“Ó¦£®| T/K | 700 | 800 | 900 | 1 000 | 1 100 |
| K | 3.3”Į10-2 | 4.71”Į10-2 | 0.10 | 2.00 | 7.87 |
| A£® | ŅŅ±½ĶŃĒā·“Ó¦ŹĒ·ÅČČ·“Ó¦ | |
| B£® | 800”ęĻĀ“ļµ½Ę½ŗāŹ±£¬±½ŅŅĻ©µÄĢå»ż·ÖŹżĪŖ40% | |
| C£® | 900”ęĻĀ“ļµ½Ę½ŗāŹ±£¬ŅŅ±½µÄ×Ŗ»ÆĀŹĪŖ50% | |
| D£® | 1 000”ęĻĀ“ļµ½Ę½ŗāŹ±£¬H2µÄÅضČĪŖ0.075 mol•L-1 |
·ÖĪö ÓɱķÖŠŹż¾ŻæÉÖŖ£¬ĪĀ¶ČŌ½øߣ¬Ę½ŗā³£ŹżŌ½“ó£¬øł¾ŻĪĀ¶Č¶ŌĘ½ŗāŅʶÆÉč²Ī¼Ó·“Ó¦µÄÓ°Ļģ·ÖĪö£»
ÉčŅŅ±½×Ŗ»ÆĮĖxmol/L
 £¬
£¬
ĘšŹ¼ÅØ¶Č£Ømol/L£©£ŗ0.2 0 0
×Ŗ»ÆÅØ¶Č£Ømol/L£©£ŗx x x
Ę½ŗāÅØ¶Č£Ømol/L£©£ŗ0.2-x x x
Ę½ŗā³£ŹżK=$\frac{c£Ø{H}_{2}£©”Įc£Ø±½ŅŅĻ©£©}{c£ØŅŅ±½£©}$£¬½įŗĻ²»Ķ¬ĪĀ¶ČĻĀµÄĘ½ŗā³£Źż¼ĘĖć£®
½ā“š ½ā£ŗA£®ÓɱķÖŠŹż¾ŻæÉÖŖ£¬ĪĀ¶ČŌ½øߣ¬Ę½ŗā³£ŹżŌ½“ó£¬ĖµĆ÷ÉżøßĪĀ¶ČĘ½ŗāĻņÕż·½ĻņŅĘ¶Æ£¬ŌņÕż·½ĻņĪŖĪüČČ·“Ó¦£¬¹ŹA“ķĪó£»
B£®800”ꏱ£¬K=4.71”Į10-2£¬ÉčŅŅ±½×Ŗ»ÆĀŹxmol/L
 £¬
£¬
ĘšŹ¼ÅØ¶Č£Ømol/L£©£ŗ0.2 0 0
×Ŗ»ÆÅØ¶Č£Ømol/L£©£ŗx x x
Ę½ŗāÅØ¶Č£Ømol/L£©£ŗ0.2-x x x
Čō±½ŅŅĻ©µÄĢå»ż·ÖŹżĪŖ40%£¬Ōņ$\frac{x}{0.2-x+x+x}$=0.4£¬½āµĆx=0.13£¬“ĖĪĀ¶ČµÄK=$\frac{c£Ø{H}_{2}£©”Įc£Ø±½ŅŅĻ©£©}{c£ØŅŅ±½£©}$=$\frac{0.13”Į0.13}{0.2-0.13}$”Ł4.71”Į10-2£¬¹ŹB“ķĪó£»
C”¢Ķ¬Ąķ900”ę£¬K=0.1£¬Ź±£¬ÉčŅŅ±½×Ŗ»ÆĀŹxmol/L ŌņK=$\frac{{x}^{2}}{0.2-x}$=0.1£¬½āµĆx=0.1£¬ŌņŅŅ±½µÄ×Ŗ»ÆĀŹĪŖ$\frac{0.1}{0.2}”Į100%$=50%£¬¹ŹCÕżČ·£»
D”¢Ķ¬Ąķ1000”ꏱ£¬K=2£¬Čō“ļµ½Ę½ŗāŹ±£¬H2µÄÅضČĪŖ0.075 mol•L-1£¬Ōņ
 £¬
£¬
Ę½ŗāÅØ¶Č£Ømol/L£©£ŗ0.2-0.075 0.075 0.075
Ōņ»ÆŃ§Ę½ŗā³£ŹżK=$\frac{0.07{5}^{2}}{0.2-0.075}$”Ł2£¬¹ŹD“ķĪó£»
¹ŹŃ”C£®
µćĘĄ ±¾ĢāµÄÖŖŹ¶µćĪŖŌĖÓĆ²»Ķ¬ĪĀ¶ČĻĀµÄĘ½ŗā³£Źż½ųŠŠ¼ĘĖć£¬ĢāÄæÄŃ¶Č²»“󣬵«ŹĒ¼ĘĖćĮæ½Ļ“ó£¬×¢ŅāŌĖÓĆ¼¼ĒÉ£¬æÉŅŌÄę·“Ė¼Ī¬¼ĘĖć£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Fe+2HClØTFeCl2+H2”ü | |
| B£® | 2HCl+Ca£ØClO£©2ØT2HClO+CaCl2 | |
| C£® | I2+2NaClO3ØT2NaIO3+Cl2”ü | |
| D£® | 4HCl£ØÅØ£©+MnO2$\frac{\underline{\;\;”÷\;\;}}{\;}$MnCl2+Cl2”ü+2H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
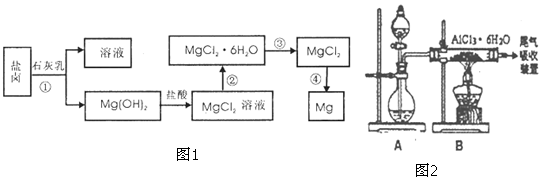
 ĀČ»ÆŃĒķæÓĆÓŚŅ½Ņ©”¢Å©Ņ©”¢Č¾ĮĻ¹¤Ņµ¼°ÓŠ»śŗĻ³É¹¤Ņµ£¬³£×÷ĀČ»Æ¼Į£¬ÖĘļ®ĀČ»ÆŃĒķæ£ØLi/SOCl2£©µē³Ų£®¹¤ŅµÉĻÓĆSO2”¢SCl2ÓėCl2·“Ó¦ŗĻ³ÉSO2£Øg£©+Cl2£Øg£©+SCl2£Øg£©?2SOCl2£Øg£©£®
ĀČ»ÆŃĒķæÓĆÓŚŅ½Ņ©”¢Å©Ņ©”¢Č¾ĮĻ¹¤Ņµ¼°ÓŠ»śŗĻ³É¹¤Ņµ£¬³£×÷ĀČ»Æ¼Į£¬ÖĘļ®ĀČ»ÆŃĒķæ£ØLi/SOCl2£©µē³Ų£®¹¤ŅµÉĻÓĆSO2”¢SCl2ÓėCl2·“Ó¦ŗĻ³ÉSO2£Øg£©+Cl2£Øg£©+SCl2£Øg£©?2SOCl2£Øg£©£®| t/min | 0 | 1 | 2 | 3 | 4 | 5 | |
| I | p | 6.0p0 | 6.7p0 | 6.1p0 | 5.4p0 | 5.0p0 | 5.0p0 |
| II | p | 6.0p0 | 7.0p0 | 5.3p0 | 5.0p0 | 5.0p0 | 5.0p0 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ¾Ż±ØµĄ£¬Ņ»¶ØĢõ¼žĻĀ Fe2O3æɱ»¼×Ķ黹ŌĪŖ”°ÄÉĆ×¼¶”±µÄ½šŹōĢś£®Ęä·“Ó¦ĪŖ£ŗFe2O3£Øs£©+3CH4£Øg£©?2Fe£Øs£©+3CO£Øg£©+6H2£Øg£©
¾Ż±ØµĄ£¬Ņ»¶ØĢõ¼žĻĀ Fe2O3æɱ»¼×Ķ黹ŌĪŖ”°ÄÉĆ×¼¶”±µÄ½šŹōĢś£®Ęä·“Ó¦ĪŖ£ŗFe2O3£Øs£©+3CH4£Øg£©?2Fe£Øs£©+3CO£Øg£©+6H2£Øg£©²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com