
| n |
| V |
| n |
| V |


 ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д�
ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д� â���̸����������������ϵ�д�
â���̸����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
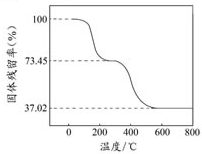 ��ʽ̼����þ[MgaAlb��OH��c��CO3��d?xH2O]������������ȼ����
��ʽ̼����þ[MgaAlb��OH��c��CO3��d?xH2O]������������ȼ����| ������Ʒ��ʣ������ |
| ������Ʒ����ʼ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ�� | �� | �� | �� | �� |
| ԭ������ | 6 | 8 | 11 | 13 |
| Ԫ�ط��� | ||||
| ���� | ||||
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Ԫ�ص���������Ԫ��ԭ���������ĵ������������Ա仯 |
| B��Ԫ�ص���������Ԫ�����ԭ�������ĵ������������Ա仯 |
| C��Ԫ�ص���������Ԫ�غ˵�����ĵ������������Ա仯 |
| D��Ԫ�ص���������Ԫ��ԭ���������ĵ������������Ա仯 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
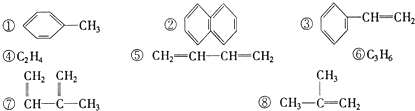
| A���١��ڡ��ݺ͢� |
| B��ȫ�� |
| C���١��ڡ��ۡ��ݡ��ߺ͢� |
| D���١��ڡ��ܡ��͢� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com