
��̼����������ָͨ��һ���ķ�������ҵ�����в�����CO2������������á�������� NaOH��Һ��������CO2���������������ͼ��ʾ����������������δ�������
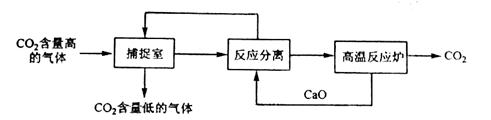
�����йظ÷���������������ȷ���ǣ� ��
A���ܺĴ��Ǹ÷�����һ��ȱ��
B������Ӧ���롱�����У�����Ļ��������������ᾧ������
C�����������У���2�����ʿ���ѭ������
D������Ӧ���롱������,�����˸��ֽⷴӦ

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ÿһ����������Ρ��������������ڱ����������ǣ����ƹ�ʹ������ϴ�·ۣ� �ڳ����������ദ�������ƹ�ʹ��һ����ľ�ʿ��ӣ� ���ƹ�ʹ�������Դ�� �ݴ���ʹ�û��ʡ�ũҩ��
A���٢ܢ� B���ڢۢ� C���٢ڢ� D���ۢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������ҹ������Ļ�ѧ�ң�1991����ȷ���In�����ԭ������Ϊ114.818�����������ԭ������ίԱ�����Ϊ�µı�ֵ�����й���In��˵���У��������( )
A. 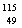 Inԭ�Ӻ�����49������ B.
Inԭ�Ӻ�����49������ B. 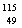 Inԭ�Ӻ�����49������
Inԭ�Ӻ�����49������
C. 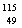 Inԭ�Ӻ�����������������֮��Ϊ115 D.
Inԭ�Ӻ�����������������֮��Ϊ115 D. 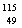 In��InԪ�ص�һ�ֺ���
In��InԪ�ص�һ�ֺ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
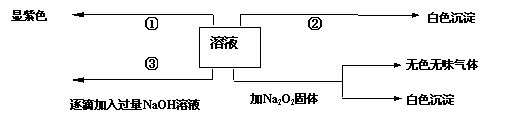
��һ��ɫ��Һ�����ܺ���Fe3+��Al3+��Fe2+��Mg2+��Cu2+��NH4+��K+��CO32-��SO42-�����ӵļ��֣�Ϊ������ɷ֣�ȡ����Һ�ֱ�������ĸ�ʵ�飬��������й���������ͼ��ʾ��
|
|
|
��ɫ����
�ڵڢ۸�ʵ���У����ɰ�ɫ�������������NaOH��������ͼ
��ʾ�����ϵ���ݴ˿�֪��
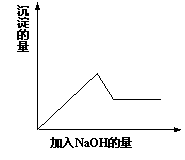 ����ԭ��Һ��һ�������ڵ�������_____________________��
����ԭ��Һ��һ�������ڵ�������_____________________��
��Ϊ�������Һ��һ�����ڵ����ӵ�Ҫ��һ����ܽ���
�ֳ�������Ϊ��д��ѧʽ��___________��_________��
��д���ڢ۸�ʵ���з�����Ӧ�����ӷ���ʽ
____________________ ___ ___
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ѧ������ܻ�ù�����߿�ѧ���������Ա�������ϡ����ȡ���۷��������Ĺ��ס���֪�������棨ϡ��Ԫ�أ��ڿ������������䰵������ʱȼ�գ���ˮ�ܿ췴Ӧ����ע���泣���Ļ��ϼ�Ϊ+3��+4�������ԣ�Ce4+>Fe3+>I2��������˵����ȷ���ǣ� ��
A�������������Ļ�ѧ����ʽ�ɱ�ʾΪ��Ce + 4HI 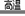 CeI4 + 2H2��
CeI4 + 2H2��
B����Ce(SO4)2��Һ�ζ�����������Һ�������ӷ���ʽΪ��Ce4+ + 2Fe2+ == Ce3+ + 2Fe3+
C������Ce����ʱ��Ӧ�ø���������ˮ
D��������ֺ���136 58Ce��138 58Ce��140 58Ce��142 58Ce�����ǻ���Ϊͬ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪����ԭ��HSO3��>I����������IO3��>I2���ں�0.3mol NaHSO3����Һ����μ���KIO3
��Һ������KIO3������I2�����ʵ����Ĺ�ϵ��������ͼ��ʾ������˵������ȷ����

A��0��b��ķ�Ӧ�����������ӷ���ʽ��ʾ��3HSO3����IO3��=3SO42����I����3H��
B��a��ʱ����NaHSO3�����ʵ���Ϊ0.12mol
C������Һ��I����I2�����ʵ���֮��Ϊ5��2ʱ�������KIO3Ϊ0.18mol
D��b��ʱ�Ļ�ԭ���������KI��NaI��b��c��Ļ�ԭ������I2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����¼������ʢ������Ȼ��� ��Ũ���� ��Cu ������ ��CH3COOH
��NaHCO3 �߾ƾ� ��Һ�壬��ջش𣺣�����ţ�
��1����������������ǿ����ʵ��� �� ���ڷǵ���ʵ��� ��
��2��д���ݢ���ˮ�еĵ��뷽��ʽ���� ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������س��õ�NaNO2����ۺ�ʳ�����ƣ�������ζ������ʹ����ʳ�ж�����֪NaNO2�ܷ������·�Ӧ��
NaNO2��HI�D��NO��I2��NaI��H2O(δ��ƽ)
(1)������Ӧ����������________������1 mol�Ļ�ԭ������������Ӧ��ת�Ƶ��ӵ���Ŀ��________��
(2)����������Ӧ�������Լ��������г��������ʽ���ʵ�飬�Լ���NaNO2��NaCl����ѡ�õ������У���ˮ���ڵ⻯�ص�����ֽ���۵��ۡ��ܰơ���ʳ�ס����м���������ʵ���________(�����)��
A���٢� B���٢ڢ�
C���٢ڢ� D���ۢ�
(3)ij������Һ�к���2%��5%��NaNO2��ֱ���ŷŻ������Ⱦ������NH4Cl��ʹNaNO2ת��Ϊ�����������Ⱦ��N2����Ӧ�Ļ�ѧ����ʽΪ___________________________
________________________________________________________________________��
(4)�����ռ�����Ṥҵβ��(NO��NO2)���Ի�ø���ƷNaNO2�����Ϊ����������a L b mol/L���ռ�����Ṥҵβ���������Ի��NaNO2�����ʵ���Ϊ________mol��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com