
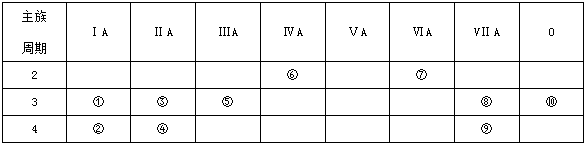
 ���õ���ʽ��ʾ�ߺ�Ԫ���⻯����γɹ�����
���õ���ʽ��ʾ�ߺ�Ԫ���⻯����γɹ�����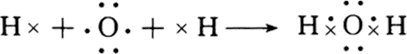 ���ߺ�Ԫ���⻯���Ԫ�آڷ�Ӧ�����ӷ���ʽ��2K+2H2O=2K++2OH-+H2����
���ߺ�Ԫ���⻯���Ԫ�آڷ�Ӧ�����ӷ���ʽ��2K+2H2O=2K++2OH-+H2�������� ��Ԫ�������ڱ���λ�ÿ�֪����ΪNa����ΪK����ΪMg����ΪCa����ΪAl����ΪC����ΪO����ΪCl����ΪBr����ΪAr��
��1��ϡ������ԭ�������Ϊ�ȶ��ṹ��Ԫ�آ���Ԫ�آ��γɵĻ�����ΪNaBr��
��2��Ԫ�آ���Ԫ�آ��γɵĻ�����ΪCCl4��
��3���ٺ�Ԫ�صĹ�������Ϊ�������ƣ��ߺ�Ԫ���⻯��Ϊˮ��Ϊ���ۻ�����ߺ�Ԫ���⻯���Ԫ�آڷ�Ӧ����KOH��������
��4��������Խǿ������������ˮ����ļ���Խǿ��
��5�����Ӳ�ṹ��ͬ���˵����Խ�����Ӱ뾶ԽС�����Ӳ�Խ�����Ӱ뾶Խ��
��� �⣺��Ԫ�������ڱ���λ�ã���֪��ΪNa����ΪK����ΪMg����ΪCa����ΪAl����ΪC����ΪO����ΪCl����ΪBr����ΪAr��
��1��ϡ������ԭ�������Ϊ�ȶ��ṹ��������ʧȥ���ӣ�Ҳ������õ��ӣ���Ar�Ļ�ѧ��������ã�Ԫ�آ���Ԫ�آ��γɵĻ�����ΪNaBr�������Ӽ���
�ʴ�Ϊ��Ar�����ӣ�
��2��Ԫ�آ���Ԫ�آ��γɵĻ�����ΪCCl4��ֻ�����ۼ���Ϊ���ۻ�����������������ܼ����ʴ�Ϊ��CCl4�����ۣ��������ܼ���
��3���ٺ�Ԫ�صĹ�������Ϊ�������ƣ������ʽΪ ���ߺ�Ԫ���⻯��Ϊˮ��Ϊ���ۻ�����õ���ʽ��ʾˮ���γɹ���Ϊ
���ߺ�Ԫ���⻯��Ϊˮ��Ϊ���ۻ�����õ���ʽ��ʾˮ���γɹ���Ϊ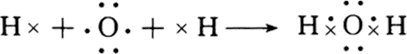 ���ߺ�Ԫ���⻯���Ԫ�آڷ�Ӧ����KOH�����������ӷ�ӦΪ2K+2H2O=2K++2OH-+H2����
���ߺ�Ԫ���⻯���Ԫ�آڷ�Ӧ����KOH�����������ӷ�ӦΪ2K+2H2O=2K++2OH-+H2����
�ʴ�Ϊ�� ��
��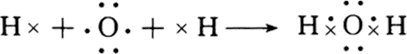 ��2K+2H2O=2K++2OH-+H2����
��2K+2H2O=2K++2OH-+H2����
��4��������ΪNa��Mg��Al������������ˮ����ļ���ΪNaOH��Mg��OH��2��Al��OH��3���ʴ�Ϊ��NaOH��Mg��OH��2��Al��OH��3��
��5�����Ӳ�ṹ��ͬ���˵����Խ�����Ӱ뾶ԽС�����Ӳ�Խ�����Ӱ뾶Խ�ʼ����ӵİ뾶��С����˳����Mg2+��Ca2+��K+���ʴ�Ϊ��Mg2+��Ca2+��K+��
���� ���⿼��λ�á��ṹ�����ʵĹ�ϵ��Ӧ�ã�Ϊ��Ƶ���㣬����Ԫ�ص�λ�á����ʡ�Ԫ�ػ�����֪ʶΪ���Ĺؼ������ط�����Ӧ�������Ŀ��飬��Ŀ�ѶȲ���


 ��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.8a g | B�� | 0.0745b g | C�� | 0.0376c g | D�� | ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
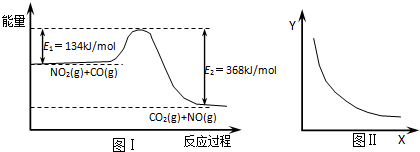
| A�� | �÷�Ӧ���ʱ��H=234kJ/mol | |
| B�� | ��X��ʾCO����ʼŨ�ȣ���Y��ʾ�Ŀ�����NO2��ת���� | |
| C�� | ��X��ʾ��Ӧʱ�䣬��Y��ʾ�Ŀ����ǻ��������ܶ� | |
| D�� | ��X��ʾ�¶ȣ���Y��ʾ�Ŀ�����CO2�����ʵ���Ũ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ȡ������Һ�������������ữ���Ȼ�����Һ���а�ɫ�������� | |
| B�� | ȡ������Һ�������Ȼ�����Һ���а�ɫ�������ɣ��ټ�ϡ�����������ʧ | |
| C�� | ȡ������Һ���������ᱵ��Һ���а�ɫ�������� | |
| D�� | ȡ������Һ�����������������ټ��Ȼ�����Һ���а�ɫ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڽ���Ԫ����ǽ���Ԫ�صķֽ��߸�������Ѱ���Ʊ��뵼����ϵ�Ԫ�� | |
| B�� | �ڹ���Ԫ���п���Ѱ���Ʊ����������º���ʴ��Ԫ�� | |
| C�� | �ڷǽ���Ԫ���������Ѱ���Ʊ�����ũҩ���ϵ�Ԫ�� | |
| D�� | ����������Ϊ8��������ϡ������Ԫ�ص�ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������ѧ��Ӧ��һ�������¶���һ������ | |
| B�� | ��ѧ��Ӧ��һ�������´ﵽ��ʱ�������淴Ӧ���ʶ������� | |
| C�� | ��ij��Ӧ��ϵ������ѹǿ���ڸı�ʱ���÷�Ӧһ���ﵽ�˷�Ӧ�� | |
| D�� | ��ij��Ӧ��һ�������´ﵽ��Ӧ��ʱ����Ӧ����������Ũ��һ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
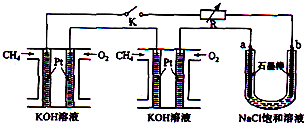
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��m-x��n | B�� | W��m-x-n��n | C�� | $\frac{W}{m}$��m-x+n�� | D�� | $\frac{m-x+n}{mW}$ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com