
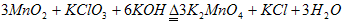
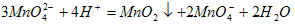
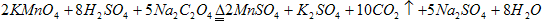


 ��ְٷְټ���ϵ�д�
��ְٷְټ���ϵ�д� �����ƻ���ĩ��̶�100��ϵ�д�
�����ƻ���ĩ��̶�100��ϵ�д� �ܿ���ȫ��100��ϵ�д�
�ܿ���ȫ��100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(12��) ʵ���ҿ������̿�(��Ҫ�ɷ�Ϊ MnO2)�Ʊ� KMnO4���������£����̿���������� KOH ��KClO3 �ڸ����·�Ӧ�����������(K2MnO4)�� KCl����ˮ�ܽ⣬��ȥ��������Һ�ữ��K2MnO4ת��ΪMnO2�� KMnO4����ȥMnO2������Ũ����Һ���ᾧ�õ�����ɫ����״KMnO4��ش�
��1�����̿��Ʊ�K2MnO4�Ļ�ѧ����ʽ�� ��
��2��K2MnO4�Ʊ�KMnO4�����ӷ���ʽ�� ��
��3����ƽ������ԭ��Ӧ����ʽ��
�ߣ� C2O42-+�ߣ�MnO4-+�ߣ�H+���ߣ�CO2+�ߣ�Mn2++�ߣ�H2O
��4����ȡ6.0 g��H2C2O4��2H2O��KHC2O4��K2SO4����������ˮ�ܽ⣬���250mL��Һ����ȡ���ݴ���Һ��25.00 mL���ֱ�����������ƿ�С�
�ٵ�һ����Һ�м����̪��Һ���μ�0.25 mol��L��1NaOH��Һ��20.00 mLʱ����Һ����ɫ��Ϊdz��ɫ������Һ���к͵�H�������ʵ���Ϊ�ߣߣ�mol��
�ڵڶ�����Һ�еμ�0.10 mol��L��1�����Ը��������Һ��16.00 mLʱ��Ӧ��ȫ����ʱ��Һ��ɫ�ɣߣ߱�Ϊ�ߣߡ�����Һ�л�ԭ�������ʵ���Ϊ�ߣߣ�mol��
��ԭ������H2C2O4��2H2O����������Ϊ�ߣߣߣߣߡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(12��) ʵ���ҿ������̿�(��Ҫ�ɷ�Ϊ MnO2)�Ʊ� KMnO4���������£����̿���������� KOH ��KClO3 �ڸ����·�Ӧ�����������(K2MnO4)�� KCl����ˮ�ܽ⣬��ȥ��������Һ�ữ��K2MnO4ת��ΪMnO2�� KMnO4����ȥMnO2������Ũ����Һ���ᾧ�õ�����ɫ����״KMnO4��ش�
��1�����̿��Ʊ�K2MnO4�Ļ�ѧ����ʽ�� ��
��2��K2MnO4�Ʊ�KMnO4�����ӷ���ʽ�� ��
��3����ƽ������ԭ��Ӧ����ʽ��
�ߣ� C2O42-+�ߣ�MnO4-+�ߣ�H+���ߣ�CO2+�ߣ�Mn2++�ߣ�H2O
��4����ȡ6.0 g��H2C2O4��2H2O��KHC2O4��K2SO4����������ˮ�ܽ⣬���250mL��Һ����ȡ���ݴ���Һ��25.00 mL���ֱ�����������ƿ�С�
�ٵ�һ����Һ�м����̪��Һ���μ�0.25 mol��L��1NaOH��Һ��20.00 mLʱ����Һ����ɫ��Ϊdz��ɫ������Һ���к͵�H�������ʵ���Ϊ�ߣߣ�mol��
�ڵڶ�����Һ�еμ�0.10 mol��L��1�����Ը��������Һ��16.00 mLʱ��Ӧ��ȫ����ʱ��Һ��ɫ�ɣߣ߱�Ϊ�ߣߡ�����Һ�л�ԭ�������ʵ���Ϊ�ߣߣ�mol��
��ԭ������H2C2O4��2H2O����������Ϊ�ߣߣߣߣߡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(12��) ʵ���ҿ������̿�(��Ҫ�ɷ�Ϊ MnO2)�Ʊ� KMnO4���������£����̿���������� KOH ��KClO3 �ڸ����·�Ӧ�����������(K2MnO4)�� KCl����ˮ�ܽ⣬��ȥ��������Һ�ữ��K2MnO4ת��ΪMnO2�� KMnO4����ȥMnO2������Ũ����Һ���ᾧ�õ�����ɫ����״KMnO4��ش�
��1�����̿��Ʊ�K2MnO4�Ļ�ѧ����ʽ�� ��
��2��K2MnO4�Ʊ�KMnO4�����ӷ���ʽ�� ��
��3����ƽ������ԭ��Ӧ����ʽ��
�ߣ� C2O42-+�ߣ�MnO4-+�ߣ�H+���ߣ�CO2+�ߣ�Mn2++�ߣ�H2O
��4����ȡ6.0 g��H2C2O4��2H2O��KHC2O4��K2SO4����������ˮ�ܽ⣬���250mL��Һ����ȡ���ݴ���Һ��25.00 mL���ֱ�����������ƿ�С�
�ٵ�һ����Һ�м����̪��Һ���μ�0.25 mol��L��1NaOH��Һ��20.00 mLʱ����Һ����ɫ��Ϊdz��ɫ������Һ���к͵�H�������ʵ���Ϊ�ߣߣ�mol��
�ڵڶ�����Һ�еμ�0.10 mol��L��1�����Ը��������Һ��16.00 mLʱ��Ӧ��ȫ����ʱ��Һ��ɫ�ɣߣ߱�Ϊ�ߣߡ�����Һ�л�ԭ�������ʵ���Ϊ�ߣߣ�mol��
��ԭ������H2C2O4��2H2O����������Ϊ�ߣߣߣߣߡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ������ѧ�����ڶ��ζο������ۺϻ�ѧ�Ծ����������� ���ͣ������
(12��) ʵ���ҿ������̿�(��Ҫ�ɷ�Ϊ MnO2)�Ʊ� KMnO4���������£����̿���������� KOH �� KClO3�ڸ����·�Ӧ�����������(K2MnO4)�� KCl����ˮ�ܽ⣬��ȥ��������Һ�ữ��K2MnO4ת��ΪMnO2�� KMnO4����ȥMnO2������Ũ����Һ���ᾧ�õ�����ɫ����״KMnO4��ش�
��1�����̿��Ʊ�K2MnO4�Ļ�ѧ����ʽ�� ��
��2��K2MnO4�Ʊ�KMnO4�����ӷ���ʽ�� ��
��3����ƽ������ԭ��Ӧ����ʽ��
�ߣ� C2O42�����ߣ�MnO4�����ߣ�H�����ߣ�CO2���ߣ�Mn2�����ߣ�H2O
��4����ȡ6.0 g��H2C2O4��2H2O��KHC2O4��K2SO4����������ˮ�ܽ⣬���250mL��Һ����ȡ���ݴ���Һ��25.00 mL���ֱ�����������ƿ�С�
�ٵ�һ����Һ�м����̪��Һ���μ�0.25 mol��L��1NaOH��Һ��20.00 mLʱ����Һ����ɫ��Ϊdz��ɫ������Һ���к͵�H�������ʵ���Ϊ�ߣߣ�mol��
�ڵڶ�����Һ�еμ�0.10 mol��L��1�����Ը��������Һ��16.00 mLʱ��Ӧ��ȫ����ʱ��Һ��ɫ�ɣߣ߱�Ϊ�ߣߡ�����Һ�л�ԭ�������ʵ���Ϊ�ߣߣ�mol��
��ԭ������H2C2O4��2H2O����������Ϊ�ߣߣߣߣߡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ������ѧ��11���¿��Ծ��������棩 ���ͣ�ʵ����
(12��) ʵ���ҿ������̿�(��Ҫ�ɷ�Ϊ MnO2)�Ʊ� KMnO4���������£����̿���������� KOH �� KClO3 �ڸ����·�Ӧ�����������(K2MnO4)�� KCl����ˮ�ܽ⣬��ȥ��������Һ�ữ��K2MnO4ת��ΪMnO2�� KMnO4����ȥMnO2������Ũ����Һ���ᾧ�õ�����ɫ����״KMnO4��ش�
��1�����̿��Ʊ�K2MnO4�Ļ�ѧ����ʽ�� ��
��2��K2MnO4�Ʊ�KMnO4�����ӷ���ʽ�� ��
��3����ƽ������ԭ��Ӧ����ʽ��
�ߣ� C2O42-+�ߣ�MnO4-+�ߣ�H+���ߣ�CO2+�ߣ�Mn2++�ߣ�H2O
��4����ȡ6.0 g��H2C2O4��2H2O��KHC2O4��K2SO4����������ˮ�ܽ⣬���250mL��Һ����ȡ���ݴ���Һ��25.00 mL���ֱ�����������ƿ�С�
�ٵ�һ����Һ�м����̪��Һ���μ�0.25 mol��L��1NaOH��Һ��20.00 mLʱ����Һ����ɫ��Ϊdz��ɫ������Һ���к͵�H�������ʵ���Ϊ�ߣߣ�mol��
�ڵڶ�����Һ�еμ�0.10 mol��L��1�����Ը��������Һ��16.00 mLʱ��Ӧ��ȫ����ʱ��Һ��ɫ�ɣߣ߱�Ϊ�ߣߡ�����Һ�л�ԭ�������ʵ���Ϊ�ߣߣ�mol��
��ԭ������H2C2O4��2H2O����������Ϊ�ߣߣߣߣߡ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com