
 ����������Ƿ�Ӧ����������ϸ������ֳ���侻��Ӧ����ʽ��ʾ��
����������Ƿ�Ӧ����������ϸ������ֳ���侻��Ӧ����ʽ��ʾ��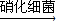 + +
+ +  ��Ũ��Ϊ mo1/L�������跴Ӧǰ����Һ��������䣩
��Ũ��Ϊ mo1/L�������跴Ӧǰ����Һ��������䣩 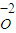 ��OԪ�ػ��ϼ���0�۽���Ϊ-2�ۣ����ϼ��ܹ�����4�ۣ����ݻ��ϼ�������ȿ�֪��NH4+ϵ��Ϊ1��O2ϵ��Ϊ2������NԪ���ǿ�֪NO3-ϵ��Ϊ1�����ݵ���غ��֪H+ϵ��Ϊ2������Ԫ���غ��֪������H2O����ϵ��Ϊ1��
��OԪ�ػ��ϼ���0�۽���Ϊ-2�ۣ����ϼ��ܹ�����4�ۣ����ݻ��ϼ�������ȿ�֪��NH4+ϵ��Ϊ1��O2ϵ��Ϊ2������NԪ���ǿ�֪NO3-ϵ��Ϊ1�����ݵ���غ��֪H+ϵ��Ϊ2������Ԫ���غ��֪������H2O����ϵ��Ϊ1�� �����ݣ�1�������ӷ���ʽ������Ҫ������������
�����ݣ�1�������ӷ���ʽ������Ҫ������������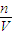 ���㣮
���㣮 ��OԪ�ػ��ϼ���0�۽���Ϊ-2�ۣ����ϼ��ܹ�����4�ۣ����ݻ��ϼ�������ȿ�֪��NH4+ϵ��Ϊ1��O2ϵ��Ϊ2������NԪ���ǿ�֪NO3-ϵ��Ϊ1�����ݵ���غ��֪H+ϵ��Ϊ2������Ԫ���غ��֪������H2O����ϵ��Ϊ1���������ӷ���ʽΪNH4++2O2=NO3-+2H++H2O��
��OԪ�ػ��ϼ���0�۽���Ϊ-2�ۣ����ϼ��ܹ�����4�ۣ����ݻ��ϼ�������ȿ�֪��NH4+ϵ��Ϊ1��O2ϵ��Ϊ2������NԪ���ǿ�֪NO3-ϵ��Ϊ1�����ݵ���غ��֪H+ϵ��Ϊ2������Ԫ���غ��֪������H2O����ϵ��Ϊ1���������ӷ���ʽΪNH4++2O2=NO3-+2H++H2O��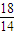 x
x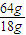 ×1mg×
×1mg× =4.57g
=4.57g ×2=0.6mol������n����NO3-��=0.2mol������NԪ���غ���n��NH4+��=n��NO3-��=0.2mol������ԭ��Һ��NH4+��Ũ��Ϊ
×2=0.6mol������n����NO3-��=0.2mol������NԪ���غ���n��NH4+��=n��NO3-��=0.2mol������ԭ��Һ��NH4+��Ũ��Ϊ =2mol/L��
=2mol/L��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��Ϋ��һģ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
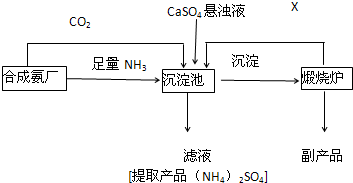
ij������Ϊ���ۺ����÷��� CaSO4 ,�����ڵĺϳɰ�������������Ʊ���NH4��2SO4 �Ĺ�������

��ش��������⣺
��1���������з�������Ҫ��Ӧ�ǣ��û�ѧ����ʽ��ʾ��
______________________________________________________
(2)����Һ�л�ȡ����NH4��2SO4���壬����IJ���������___________________(��д���)
A.�������� B.����Ũ�� C.��ȴ�ᾧ D.���� E.��ȡ F��Һ
��3�������������п���ѭ��ʹ�õ�X��__________________.
��4���������У�Ҫ������Ͱ����Ĺܵ��Ƿ�©������ѡ��____________(��д���)
A.Ũ���� B.ʪ�����ɫʯ����ֽ C.ʪ��ĵ��۵⻯����ֽ D.ϡ���� E.ʪ��ĺ�ɫʯ����ֽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij������Ϊ���ۺ����÷��� CaSO4 ,�����ڵĺϳɰ�������������Ʊ���NH4��2SO4 �Ĺ�������
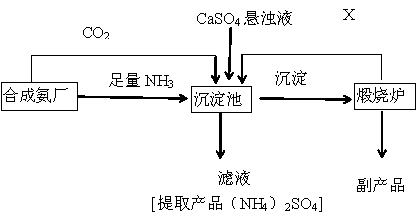
��ش��������⣺
��1���������з�������Ҫ��Ӧ�ǣ��û�ѧ����ʽ��ʾ��
______________________________________________________
(2)����Һ�л�ȡ����NH4��2SO4���壬����IJ���������___________________(��д���)
A.�������� B.����Ũ�� C.��ȴ�ᾧ D.���� E.��ȡ F��Һ
��3�������������п���ѭ��ʹ�õ�X��__________________.
��4���������У�Ҫ������Ͱ����Ĺܵ��Ƿ�©������ѡ��____________(��д���)
A.Ũ���� B.ʪ�����ɫʯ����ֽ C.ʪ��ĵ��۵⻯����ֽ D.ϡ���� E.ʪ��ĺ�ɫʯ����ֽ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com