
| 40m2 |
| 100 |
| ||
| m1 |
| 40m2 |
| 100m1 |
| 40m2 |
| 100m1 |



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĪŹ“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĪŹ“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ÓĆĢģĘ½×¼Č·³ĘČ”19.1gĢ¼ĖįÄĘ¹ĢĢå |
| B£®½«¹ĢĢåŌŚÉÕ±ÖŠČܽāŗó£¬Ó¦Į¢¼“½«ČÜŅŗ×ŖŅĘÖĮČŻĮæĘæÖŠ |
| C£®ÉÕ±ÖŠµÄČÜŅŗ×ŖŅĘŗó£¬ÓÉÓŚ²ŠĮōĮæŗÜÉŁ£¬æÉŅŌ²»±ŲŌŁĻ“µÓÉÕ±ŗĶ²£Į§°ō |
| D£®Čē¹ūŌŚ×īŗó¶ØČŻŹ±£¬µĪČėµÄĖ®³¬¹żĮĖČŻĮæĘæµÄæĢ¶ČĻߣ¬ŹµŃé±ŲŠėÖŲ×ö |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĪŹ“šĢā
| ·“Ó¦½×¶Ī | ¢ń | ¢ņ | ¢ó |
| ŃĪĖįĢå»żx/mL | 0£¼x”Ü10.0 | 10.0£¼x”Ü40.0 | x£¾40.0 |
| ĻÖĻó | ĪŽĘųĢå | ÓŠĘųĢå | ĪŽĘųĢå |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĪŹ“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĪŹ“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
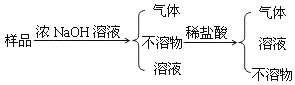
| A£®Al”¢Mg£ØOH£©2”¢S”¢Cu | B£®Al”¢Fe”¢Mg”¢Cu |
| C£®Al2O3”¢Fe”¢C”¢Cu | D£®Al”¢C”¢CuO”¢Cu |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com