

���� ��1��Feԭ�Ӻ��������Ϊ26�������������ԭ����д��������Ų�ʽ��
��2�������Fe��CO��x���۷е�Ƚϵͣ������ڷǼ����ܼ���Ӧ���ڷ��Ӿ��壻
Feԭ�Ӽ۵�����Ϊ8��CO�ṩһ�Թ¶Ե��ӣ���Feԭ���γ���λ����
������ΪFe��CO��Fe���ڽ������壬���н�������CO�����к��й��ۼ���
��3��K3[Fe��CN��6]��Һ���������ӷ�Ӧ�õ���ɫ�����������ڼ����������ӣ�
CN-��N2����ȵ����壬CN-��Cԭ����Nԭ��֮���γɶԹ��õ��Ӷԣ����й¶Ե��ӣ��ӻ������ĿΪ2��
ͬ������ԭ����������Ԫ�ص�һ�����ܳ��������ƣ���Ԫ��2p�ܼ�Ϊ�����ȶ�״̬����һ�����ܸ���ͬ��������Ԫ�صģ�
��4����ͭԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d104s1��
��Cu�ľ����ѻ���ʽΪ���������ܶѻ�����λ��Ϊ12��
��5����Mԭ�ӵ���Χ�����Ų�ʽΪ3s23p5����MΪCl�����ݾ�̯�����㾧����Cuԭ�ӡ�Clԭ����Ŀ������ȷ����ѧʽ��
�ڵ縺�Բ����1.7�ļ�һ�������Ӽ���С��1.7��һ��Ϊ���ۼ���
��Cuԭ������Χ��4��Clԭ���γ���������ṹ�������������ĵ�Cu�뾧������Clԭ�Ӿ�����̣����ǵ����ߴ��ھ�����Խ����ϣ���Ϊ������Խ��߳��ȵ�$\frac{1}{4}$�����ݾ�������ԭ����Ŀ���㾧������������ܶȼ��㾧��������������㾧���ⳤ��������Խ��߳���Ϊ�ⳤ��$\sqrt{3}$����
��� �⣺��1��Feԭ�Ӻ��������Ϊ26�������������ԭ������������Ų�ʽΪ��1s22s22p63s23p63d64s2��
�ʴ�Ϊ��1s22s22p63s23p63d64s2��
��2�������Fe��CO��x���۷е�Ƚϵͣ������ڷǼ����ܼ���Ӧ���ڷ��Ӿ��壻Feԭ�Ӽ۵�����Ϊ8��CO�ṩһ�Թ¶Ե��ӣ���Feԭ���γ���λ������8+2x=18�����x=5��������ΪFe��CO��Fe���ڽ������壬���н�������CO�����к��й��ۼ���
�ʴ�Ϊ�����Ӿ��壻5�������������ۼ���
��3��K3[Fe��CN��6]��Һ���������ӷ�Ӧ�õ���ɫ�����������ڼ���Fe2+���ӣ�
CN-��N2����ȵ����壬CN-��Cԭ����Nԭ��֮���γɶԹ��õ��Ӷԣ�����1�Թ¶Ե��ӣ��ӻ������ĿΪ2��Cԭ�Ӳ�ȡsp�ӻ���
ͬ������ԭ����������Ԫ�ص�һ�����ܳ��������ƣ���Ԫ��2p�ܼ�Ϊ�����ȶ�״̬����һ�����ܸ���ͬ��������Ԫ�صģ��ʵ�һ�����ܣ�N��O��C��
�ʴ�Ϊ��Fe2+��sp�ӻ���N��O��C��
��4����ͭԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d104s1���������ڱ��У��������ڵڢ�B�壬
�ʴ�Ϊ���������ڵڢ�B�壻
��Cu�ľ����ѻ���ʽΪ���������ܶѻ�����λ��Ϊ12����ÿ��ͭԭ����Χ���������ͭԭ����ĿΪ12��
�ʴ�Ϊ��12��
��5����Mԭ�ӵ���Χ�����Ų�ʽΪ3s23p5����MΪCl��������Cuԭ��Ϊ4��Clԭ����ĿΪ8��$\frac{1}{8}$+6��$\frac{1}{2}$=4���ʻ�ѧʽΪCuCl��
�ʴ�Ϊ��CuCl��
��ͭ��M�ĵ縺�����3.0-1.9=1.1���縺�Բ����1.7�ļ�һ�������Ӽ���С��1.7��һ��Ϊ���ۼ�����Cu��M�γɵĻ��������ڹ��ۻ����
�ʴ�Ϊ�����ۣ�
��Cuԭ������Χ��4��Clԭ���γ���������ṹ�������������ĵ�Cu�뾧������Clԭ�Ӿ�����̣����ǵ����ߴ��ھ�����Խ����ϣ���Ϊ������Խ��߳��ȵ�$\frac{1}{4}$����������Ϊ$\frac{4��99.5}{{N}_{A}}$g�����ⳤΪ$\root{3}{\frac{\frac{4��99.5}{{N}_{A}}g}{��g•c{m}^{-3}}}$=$\root{3}{\frac{4��99.5}{��{N}_{A}}}$cm��������Խ��߳���Ϊ�ⳤ��$\sqrt{3}$������þ�����ͭԭ�Ӻ�Mԭ��֮�����̾���Ϊ$\frac{1}{4}$��$\sqrt{3}$��$\root{3}{\frac{4��99.5}{��{N}_{A}}}$cm����Ϊ$\frac{\sqrt{3}}{4}$��$\root{3}{\frac{4��99.5}{��{N}_{A}}}$��1010pm��
�ʴ�Ϊ��$\frac{\sqrt{3}}{4}$��$\root{3}{\frac{4��99.5}{��{N}_{A}}}$��1010��
���� �����Ƕ����ʽṹ�����ʵĿ��飬�漰�������Ų�ʽ�������ܡ��縺�ԡ��ӻ����͵��жϡ������������㣬��5���Т�Ϊ�״��㡢�ѵ㣬��ѧ���߱�һ���Ŀռ���������ѧ��������Ŀ�ѶȽϴ�


 ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д� Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų����������ش��������⣺
��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų����������ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Si��P��S��ClԪ�صĵ�������������Խ��Խ���� | |
| B�� | �ǽ���Ԫ�صķǽ�����Խǿ�����������Ӧˮ���������Ҳһ��Խǿ | |
| C�� | Ԫ��ԭ������������Խ�࣬Ԫ�ؽ�����Խǿ | |
| D�� | F-��O2-��Mg2+��Na+���Ӱ뾶��С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1.0Ll.0mol��L-1��NaAlO2ˮ��Һ�к��е���ԭ����Ϊ2NA | |
| B�� | ��ϩ�ͻ�������ɵ�42g�����������ԭ�ӵĸ���Ϊ6NA | |
| C�� | 25CʱpH=11��Na2C03��Һ��ˮ�������OH-����ĿΪ0.001NA | |
| D�� | ��ԭ������Ϊ0.2NA��S02��O2�Ļ�����壬�����Ϊ2.24L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
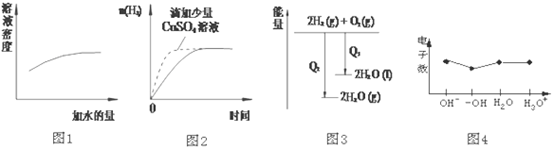
| A�� | ͼ1��ʾŨ�����ϡ�� | |
| B�� | ͼ2��ʾ�����������������п�۷�Ӧ | |
| C�� | ͼ3��ʾ������������Ӧ�е������仯 | |
| D�� | ͼ4��ʾ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ba2+ | B�� | K+ | C�� | Mg2+ | D�� | HCO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����--SiO2 | B�� | ����--Al2��SO4��3•12H2O | ||
| C�� | С�մ�--Na2CO3 | D�� | Ư��--Ca��ClO��2��CaCl2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com