


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������ȼ��Ч�ʣ���Լ��Դ�ǵ������������Դ�о���ǰ�ؿ���֮һ |
| B������ȼ��һ���Ƿ��ȷ�Ӧ |
| C����ɫֲ����й������ʱ̫����ת��Ϊ��ѧ�� |
| D������������¯�ĸ߶ȣ����Խ���¯����CO�ĺ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ڳ��³�ѹ�£�11.2LN2���еķ�����ΪNA |
| B��32gO2�ڱ�״������ռ���ԼΪ22.4L |
| C����״���£�18gH2O��ռ�����Լ��22.4L |
| D����ͬ��ͬѹ�£���ͬ������κ����嵥��������ԭ������ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1��ͼI��ʾ10mL��Ͳ��Һ���λ�ã�A��B��B��C�̶ȼ����1mL������̶�AΪ6����Ͳ��Һ������Ϊ
��1��ͼI��ʾ10mL��Ͳ��Һ���λ�ã�A��B��B��C�̶ȼ����1mL������̶�AΪ6����Ͳ��Һ������Ϊ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
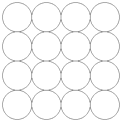 ijͬѧ��ѧϰ�Ⱦ������ܶѻ����������ܶѻ�A1���������ܶѻ�A3�����������һ�����ܶѻ���ʽAx����ͼ��ʾΪAx�ѻ���Ƭ����ʽ��Ȼ��ڶ���Ͷѻ��ڵ�һ��Ŀ�϶�ϣ������Ax�Ķѻ���ʽ�ش𣺣���Ҫд�����̣�
ijͬѧ��ѧϰ�Ⱦ������ܶѻ����������ܶѻ�A1���������ܶѻ�A3�����������һ�����ܶѻ���ʽAx����ͼ��ʾΪAx�ѻ���Ƭ����ʽ��Ȼ��ڶ���Ͷѻ��ڵ�һ��Ŀ�϶�ϣ������Ax�Ķѻ���ʽ�ش𣺣���Ҫд�����̣��鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com