
ŅųĶŗĻ½š¹ć·ŗÓĆÓŚŗ½æÕ¹¤Ņµ”£“ÓĒŠøī·ĻĮĻÖŠ»ŲŹÕŅų²¢ÖʱøĶ»Æ¹¤²śĘ·µÄ¹¤ŅÕČēĻĀ£ŗ
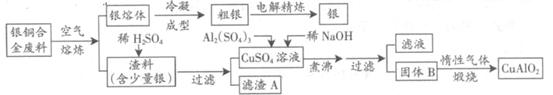
£Ø×¢£ŗAl(OH)3ŗĶCu(OH)2æŖŹ¼·Ö½āµÄĪĀ¶Č·Ö±šĪŖ450”ęŗĶ80”ę£©
£Ø1£©µē½ā¾«Į¶ŅųŹ±£¬Ņõ¼«·“Ó¦Ź½ĪŖ £»ĀĖŌüAÓėĻ”HNO3·“Ó¦£¬²śÉśµÄĘųĢåŌŚæÕĘųÖŠŃøĖŁ±äĪŖŗģ×ŲÉ«£¬øĆĘųĢå±äÉ«µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø2£©¹ĢĢå»ģŗĻĪļBµÄ×é³ÉĪŖ £»ŌŚÉś³É¹ĢĢåBµÄ¹ż³ĢÖŠ£¬ŠčæŲÖĘNaOHµÄ¼ÓČėĮ棬ČōNaOH¹żĮ棬ŌņŅņ¹żĮæŅżĘšµÄ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”£
£Ø3£©Ķź³ÉģŃÉÕ¹ż³ĢÖŠŅ»øö·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ CuO+ Al2O3 CuAlO2 + ”ü”£
CuAlO2 + ӟӣ
£Ø4£©ČōŅųĶŗĻ½šÖŠĶµÄÖŹĮæ·ÖŹżĪŖ63.5%£¬ĄķĀŪÉĻ5.0kg·ĻĮĻÖŠµÄĶæÉĶźČ«×Ŗ»ÆĪŖ mol CuAlO2£¬ÖĮÉŁŠčŅŖ1.0mol•L—1µÄAl2(SO4)3ČÜŅŗ L”£
£Ø5£©CuSO4ČÜŅŗŅ²æÉÓĆÓŚÖʱøµØ·Æ£¬Ę仳±¾²Ł×÷ŹĒ ”¢¹żĀĖ”¢Ļ“µÓŗĶøÉŌļ”£
 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚŌŹŠķ¼ÓČȵÄĢõ¼žĻĀ£¬Ö»ÓĆŅ»ÖÖŹŌ¼Į¾ĶæÉŅŌ¼ų±šĀČ»Æ¼Ų”¢Ģ¼Ėį¼Ų”¢Ę«ĀĮĖį¼Ų”¢ĒāŃõ»Æ¼Ų”¢ĒāŃõ»Æ±µ”¢ĀČ»Æ±µ6ÖÖČÜŅŗ£¬ÕāÖÖŹŌ¼ĮŹĒ£Ø””””£©
A.H2SO4”””””””””””””” ””B.£ØNH4£©2SO4
C.NH3·H2O D.NH4HSO4
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ¶ŌÓŠ»śĪļ½į¹¹»ņŠŌÖŹµÄĆčŹö£¬ÕżČ·µÄŹĒ£Ø £©
A£®¼×±½ÖŠŗ¬ÓŠ ÉŁĮæ±½·Ó£¬æÉ¼ÓŹŹĮæÅØäåĖ®£¬ŌŁ¹żĀĖ¶ų³żČ„
ÉŁĮæ±½·Ó£¬æÉ¼ÓŹŹĮæÅØäåĖ®£¬ŌŁ¹żĀĖ¶ų³żČ„
B£®±½µÄ¼ä¶žäå“śĪļĪŽĶ¬·ÖŅģ¹¹ĢåÄÜĖµĆ÷±½²»ŹĒµ„Ė«¼ü½»Ģę½į¹¹
C£®Ź¹ÓĆĖįŠŌKMnO4ČÜŅŗ³żČ„ŅŅĶéÖŠ»ģÓŠµÄŅŅĻ©
D£®Ņ»¶ØĢõ¼žĻĀ£¬Cl2ŌŚ¼×±½µÄ±½»·»ņ²ąĮ“ÉĻ¾łÄÜ·¢ÉśČ”“ś·“Ó¦
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
LiPF6ŹĒļ®Ąė×Óµē³ŲÖŠ¹ć·ŗÓ¦ÓƵĵē½āÖŹ”£Ä³¹¤³§ÓĆLiF”¢PCl5ĪŖŌĮĻ£¬µĶĪĀ·“Ó¦ÖʱøLiPF6£¬ĘäĮ÷³ĢČēĻĀ£ŗ
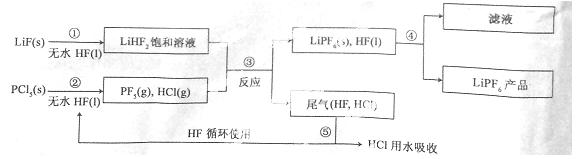
ŅŃÖŖ£ŗHClµÄ·ŠµćŹĒ£85.0 ”ę£¬HFµÄ·ŠµćŹĒ19.5 ”ę”£
£Ø1£©µŚ¢Ł²½·“Ó¦ÖŠĪŽĖ®HFµÄ×÷ÓĆŹĒ ”¢ ”£·“Ó¦Éč±ø²»ÄÜÓĆ²£Į§²ÄÖŹµÄŌŅņŹĒ (ÓĆ»Æѧ·½³ĢŹ½±ķŹ¾)”£ĪŽĖ®HFÓŠøÆŹ“ŠŌŗĶ¶¾ŠŌ£¬¹¤³§°²Č«ŹÖ²įĢįŹ¾£ŗČē¹ū²»Š”ŠÄ½«HFÕ“µ½Ę¤·ōÉĻ£¬æÉĮ¢¼“ÓĆ2%µÄ ČÜŅŗ³åĻ“”£
£Ø2£©øĆĮ÷³ĢŠčŌŚĪŽĖ®Ģõ¼žĻĀ½ųŠŠ£¬µŚ¢Ū²½·“Ó¦ÖŠPCl5¼«Ņ×Ė®½ā£¬Ęä²śĪļĪŖĮ½ÖÖĖį£¬Š“³öPF5Ė®½āµÄ»Æѧ·½³ĢŹ½£ŗ ”£
£Ø3£©µŚ¢Ü²½·ÖĄė²ÉÓƵķ½·ØŹĒ £»µŚ¢Ż²½·ÖĄėĪ²ĘųÖŠHF”¢HCl²ÉÓƵķ½·ØŹĒ ”£
£Ø4£©LiPF6²śĘ·ÖŠĶس£»ģÓŠÉŁĮæLiF”£Č”ѳʷwg£¬²āµĆLiµÄĪļÖŹµÄĮæĪŖnmol£¬ŌņøĆѳʷ֊LiPF6µÄĪļÖŹµÄĮæĪŖ mol(ÓĆŗ¬ÓŠw”¢nµÄ“śŹżŹ½±ķŹ¾)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ńõ»ÆĆ¾ŌŚŅ½Ņ©”¢½ØÖžµČŠŠŅµÓ¦ÓĆ¹ć·ŗ”£ĮņĖįĆ¾»¹ŌČČ½āÖʱøøß“æŃõ»ÆĆ¾ŹĒŅ»ÖÖŠĀµÄĢ½Ė÷”£ŅŌĮāĆ¾æó(Ö÷ŅŖ³É·ÖĪŖMgCO3£¬ŗ¬ÉŁĮæFeCO3 )ĪŖŌĮĻÖʱøøß“æŃõ»ÆĆ¾µÄŹµŃéĮ÷³ĢČēĻĀ£ŗ
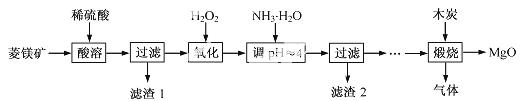
£Ø1£©MgCO3 ÓėĻ”ĮņĖį·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”£
£Ø2£©¼ÓČėH2O2 Ńõ»ÆŹ±£¬·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø3£©ĀĖŌü2 µÄ³É·ÖŹĒ (Ģī»ÆѧŹ½)”£
|
|
|
MgSO4+3C=== === MgO+Sӟ+3COӟ
ĄūÓĆÓŅĶ¼×°ÖƶŌģŃÉÕ²śÉśµÄĘųĢå½ųŠŠ·Ö²½ĪüŹÕ»ņŹÕ¼Æ”£
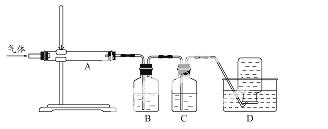
¢ŁDÖŠŹÕ¼ÆµÄĘųĢåæÉŅŌŹĒ (Ģī»ÆѧŹ½)”£
¢ŚBÖŠŹ¢·ÅµÄČÜŅŗæÉŅŌŹĒ (Ģī×ÖÄø)”£
a£®NaOH ČÜŅŗ b£®Na2CO3 ČÜŅŗ c£®Ļ”ĻõĖį d£®KMnO4ČÜŅŗ
¢ŪAÖŠµĆµ½µÄµ»ĘÉ«¹ĢĢåÓėČȵÄNaOHČÜŅŗ·“Ó¦£¬²śĪļÖŠŌŖĖŲ×īøß¼ŪĢ¬ĪŖ+4£¬Š“³öøĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ  ”£
ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
»ÆѧŹµŃéŹŅĶس£ÓĆ“ÖŠæŗĶĻ”ĮņĖį·“Ó¦ÖĘĒāĘų£¬Ņņ“ĖŌŚÖĘĒā·ĻŅŗÖŠŗ¬ÓŠ“óĮæµÄĮņĖįŠæ”£Ķ¬Ź±£¬ÓÉÓŚ“ÖŠæÖŠ»¹ŗ¬ÓŠĢśµČŌÓÖŹ£¬Ź¹µĆČÜŅŗÖŠ»ģÓŠŅ»¶ØĮæµÄĮņĖįŃĒĢś£¬ĪŖĮĖ³ä·ÖĄūÓĆÖĘĒā·ĻŅŗ£¬³£ÓĆĘäÖʱøš©·Æ(ZnSO4·7H2O)”£Ä³Š£»ÆѧŠĖȤŠ”×éµÄĶ¬Ń§ŅŌÖĘĒāĘųµÄ·ĻŅŗĪŖŌĮĻĄ“ÖĘČ”š©·Æ”£Öʱøš©·ÆµÄŹµŃéĮ÷³ĢČēĻĀĶ¼ĖłŹ¾”£

ŅŃÖŖ£ŗæŖŹ¼Éś³ÉĒāŃõ»ÆĪļ³Įµķµ½³ĮµķĶźČ«µÄpH·¶Ī§·Ö±šĪŖFe(OH)3£ŗ2.7”«3.7£»Fe(OH)2£ŗ7.6”«9.6£»Zn(OH)2£ŗ5.7”«8.0£¬ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)¼ÓČėµÄŹŌ¼Į¢Ł£¬¹©Ń”ŌńŹ¹ÓƵÄÓŠ£ŗ°±Ė®”¢NaClOČÜŅŗ”¢20%µÄH2O2”¢ÅØĮņĖį”¢ÅØĻõĖįµČ£¬×īŗĆŃ”ÓĆ________£¬ĘäĄķÓÉŹĒ
______________________________________________________________________ӣ
(2)¼ÓČėµÄŹŌ¼Į¢Ś£ŗ¹©Ń”ŌńŹ¹ÓƵÄÓŠ£ŗa.Zn·Ū”¢b.ZnO”¢c.Zn(OH)2”¢d.ZnCO3”¢e.ZnSO4µČ£¬æÉŃ”ÓĆ__________”£
(3)“Ó¾§Ģå1”ś¾§Ģå2£¬øĆ¹ż³ĢµÄĆū³ĘŹĒ__________”£
(4)ŌŚµĆµ½š©·ÆŹ±£¬Ļņ¾§ĢåÖŠ¼ÓČėÉŁĮæ¾Ę¾«Ļ“µÓ¶ų²»ÓĆĖ®µÄŌŅņŹĒ_____________________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ·“Ó¦ŹōÓŚČ”“ś·“Ó¦µÄŹĒ(””””)
A£®ŅŅĻ©ĶØČėĖįŠŌøßĆĢĖį¼ŲČÜŅŗÖŠ
B£®ŅŅĻ©ĶØČėäåĖ®ÖŠ
C£®ŌŚÄų×÷“߻ƼĮµÄĢõ¼žĻĀ£¬±½ÓėĒāĘų·“Ó¦
D£®±½ÓėŅŗäå»ģŗĻŗó¼Ó ČėĢś·Ū
ČėĢś·Ū
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijŗ¬±½»·µÄ»ÆŗĻĪļA£¬ĘäĻą¶Ō·Ö×ÓÖŹĮæĪŖ104£¬Ģ¼µÄÖŹĮæ·ÖŹżĪŖ92.3%.
£Ø1£©AµÄ·Ö×ÓŹ½ĪŖ_____________________________________________________£»
£Ø2£©AÓėäåµÄĖÄĀČ»ÆĢ¼ČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ___________________£¬·“Ó¦ĄąŠĶŹĒ________£»
£Ø3£©ŅŃÖŖ

ĒėŠ“³öAÓėĻ””¢ĄäµÄKMnO4ČÜŅŗŌŚ¼īŠŌĢõ¼žĻĀ·“Ó¦µÄ»Æѧ·½³ĢŹ½__________________________£»
£Ø4£©ŌŚŅ»¶ØĢõ¼žĻĀ£¬AÓėĒāĘų·“Ó¦£¬µĆµ½µÄ»ÆŗĻĪļÖŠĢ¼µÄÖŹĮæ·ÖŹżĪŖ85.7%.Š“³ö“Ė»ÆŗĻĪļµÄ½į¹¹¼ņŹ½____________________________________________________________£»
 £Ø5£©ŌŚŅ»¶ØĢõ¼žĻĀ£¬ÓÉA¾ŪŗĻµĆµ½µÄøß·Ö×Ó»ÆŗĻĪļµÄ½į¹¹¼ņŹ½ĪŖ________________£®
£Ø5£©ŌŚŅ»¶ØĢõ¼žĻĀ£¬ÓÉA¾ŪŗĻµĆµ½µÄøß·Ö×Ó»ÆŗĻĪļµÄ½į¹¹¼ņŹ½ĪŖ________________£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijӊ»śĪļ1.6gŌŚ×ćĮæµÄŃõĘųÖŠČ¼ÉÕŗó²āµĆÉś³É¶žŃõ»ÆĢ¼µÄÖŹĮæĪŖ4.4g£¬Ė®µÄÖŹĮæĪŖ3.6gŌņ £Ø £©
A.ÓŠ»śĪļŅ»¶Øŗ¬C,HŌŖĖŲ£¬æÉÄÜŗ¬ŃõŌŖĖŲ B.ÓŠ»śĪļÖ»ŗ¬C,HŌŖĖŲ
C . ÓŠ»śĪļŗ¬C,H,OŌŖĖŲ D.
. ÓŠ»śĪļŗ¬C,H,OŌŖĖŲ D. “ĖÓŠ»śĪļ»ÆѧŹ½æÉÄÜĪŖCH4
“ĖÓŠ»śĪļ»ÆѧŹ½æÉÄÜĪŖCH4
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com