
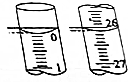
 Ä³Ń§ÉśÓĆŅŃÖŖĪļÖŹµÄĮæÅØ¶ČµÄŃĪĖįĄ“²ā¶ØĪ“ÖŖĪļÖŹµÄĮæÅØ¶ČµÄNaOHČÜŅŗŹ±£¬Ń”Ōń·ÓĢŖ×÷ÖøŹ¾¼Į£®ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ
Ä³Ń§ÉśÓĆŅŃÖŖĪļÖŹµÄĮæÅØ¶ČµÄŃĪĖįĄ“²ā¶ØĪ“ÖŖĪļÖŹµÄĮæÅØ¶ČµÄNaOHČÜŅŗŹ±£¬Ń”Ōń·ÓĢŖ×÷ÖøŹ¾¼Į£®ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ| µĪ¶Ø“ĪŹż | “ż²āNaOHČÜŅŗµÄĢå»ż/mL | 0.1000mol•L-1ŃĪĖįµÄĢå»ż/mL | ||
| µĪ¶ØĒ°æĢ¶Č | µĪ¶ØŗóæĢ¶Č | ČÜŅŗĢå»ż/mL | ||
| µŚŅ»“Ī | 25.00 | 0.00 | 26.11 | 26.11 |
| µŚ¶ž“Ī | 25.00 | 1.56 | 30.30 | 28.74 |
| µŚČż“Ī | 25.00 | 0.22 | 26.31 | 26.09 |
·ÖĪö £Ø1£©Ėį¼īÖŠŗĶµĪ¶ØŹ±£¬ŃŪ¾¦ŅŖ×¢ŹÓ׶ŠĪĘæÄŚČÜŅŗµÄŃÕÉ«±ä»Æ£»ŅŃÖŖĪļÖŹµÄĮæÅØ¶ČµÄŃĪĖįĄ“²ā¶ØĪ“ÖŖĪļÖŹµÄĮæÅØ¶ČµÄNaOHČÜŅŗŹ±£¬Ń”Ōń·ÓĢŖ×÷ÖøŹ¾¼Į£¬µĪ¶ØÖÕµćŹ±ČÜŅŗŃÕÉ«ÓÉŗģÉ«Ķ»±äĪŖĪŽÉ«£¬ĒŅ°ė·ÖÖÓÄŚ²»ĶŹÉ«£»
£Ø2£©øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©”ĮV£Ø±ź×¼£©}{V£Ø“ż²ā£©}$·ÖĪö²»µ±²Ł×÷¶ŌV£Ø±ź×¼£©µÄÓ°Ļģ£¬ŅŌ“ĖÅŠ¶ĻÅØ¶ČµÄĪó²ī£»
£Ø3£©øł¾ŻµĪ¶Ø¹ÜµÄ½į¹¹ŗĶ¾«Č·¶ČŅŌ¼°²āĮæµÄŌĄķ¶ĮŹż£»
£Ø4£©ŅĄ¾ŻĶ¼±ķŹż¾Ż£¬ĻČøł¾ŻŹż¾ŻµÄÓŠŠ§ŠŌ£¬ÉįČ„µŚ2×鏿¾Ż£¬Č»ŗóĒó³ö1”¢3×éĘ½¾łĻūŗÄV£ØŃĪĖį£©£¬½Ó×Åøł¾ŻŃĪĖįŗĶNaOH·“Ó¦Ēó³öc£ØNaOH£©£®
½ā“š ½ā£ŗ£Ø1£©Ėį¼īÖŠŗĶµĪ¶ØŹ±£¬ŃŪ¾¦ŅŖ×¢ŹÓ׶ŠĪĘæÄŚČÜŅŗµÄŃÕÉ«±ä»Æ£¬ŅŌÅŠ¶ĻÖÕµć£¬ŃĪĖįŗĶĒāŃõ»ÆÄĘĒ”ŗĆ·“Ó¦ČÜŅŗ³ŹÖŠŠŌ£¬Ń”Ōń¼īŠŌ±äÉ«·¶Ī§ÄŚµÄÖøŹ¾¼Į·ÓĢŖ£¬µĪ¶ØÖÕµćŹ±ČÜŅŗŃÕÉ«ÓÉŗģÉ«Ķ»±äĪŖĪŽÉ«£¬ĒŅ°ė·ÖÖÓÄŚ²»ĶŹÉ«£»
¹Ź“š°øĪŖ£ŗ׶ŠĪĘæÄŚČÜŅŗµÄŃÕÉ«±ä»Æ£»°ė·ÖÖÓÄŚ²»ĶŹÉ«£»
£Ø2£©A£®ĖįŹ½µĪ¶Ø¹ÜĪ“ÓƱź×¼ŃĪĖįČÜŅŗČóĻ“¾ĶÖ±½Ó×¢Čė±ź×¼ŃĪĖįČÜŅŗ£¬±ź×¼ŅŗµÄÅضČĘ«Š”£¬Ōģ³ÉV£Ø±ź×¼£©Ę«“ó£¬øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©”ĮV£Ø±ź×¼£©}{V£Ø“ż²ā£©}$·ÖĪö£¬²ā¶Øc£Ø“ż²ā£©Ę«“󣬹ŹA“ķĪó£»
B£®µĪ¶ØĒ°Ź¢·ÅĒāŃõ»ÆÄĘČÜŅŗµÄ׶ŠĪĘæÓĆÕōĮóĖ®Ļ“¾»ŗóƻӊøÉŌļ£¬“ż²āŅŗµÄĪļÖŹµÄĮæ²»±ä£¬¶ŌV£Ø±ź×¼£©ĪŽÓ°Ļģ£¬øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©”ĮV£Ø±ź×¼£©}{V£Ø“ż²ā£©}$·ÖĪö£¬²ā¶Øc£Ø“ż²ā£©ĪŽÓ°Ļģ£¬¹ŹB“ķĪó£»
C£®ĖįŹ½µĪ¶Ø¹ÜŌŚµĪ¶ØĒ°ÓŠĘųÅŻ£¬µĪ¶ØŗóĘųÅŻĻūŹ§£¬Ōģ³ÉV£Ø±ź×¼£©Ę«“ó£¬øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©”ĮV£Ø±ź×¼£©}{V£Ø“ż²ā£©}$·ÖĪö£¬²ā¶Øc£Ø“ż²ā£©Ę«“󣬹ŹC“ķĪó£»
D£®¶ĮČ”ŃĪĖįĢå»żŹ±£¬æŖŹ¼ŃöŹÓ¶ĮŹż£¬µĪ¶Ø½įŹųŹ±ø©ŹÓ¶ĮŹż£¬Ōģ³ÉV£Ø±ź×¼£©Ę«Š”£¬øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©”ĮV£Ø±ź×¼£©}{V£Ø“ż²ā£©}$·ÖĪö£¬²ā¶Øc£Ø“ż²ā£©Ę«Š”£¬¹ŹDÕżČ·£»
¹ŹŃ”D£»
£Ø3£©ĘšŹ¼¶ĮŹżĪŖ0.00mL£¬ÖÕµć¶ĮŹżĪŖ26.10mL£¬ŃĪĖįČÜŅŗµÄĢå»żĪŖ26.10mL£¬¹Ź“š°øĪŖ£ŗ0.00£»26.10£»26.10£»
£Ø4£©øł¾ŻŹż¾ŻµÄÓŠŠ§ŠŌ£¬ÉįČ„µŚ2×鏿¾Ż£¬Ōņ1”¢3×éĘ½¾łĻūŗÄV£ØŃĪĖį£©=$\frac{26.11mL+26.09mL}{2}$=26.10mL£¬
øł¾Ż·“Ó¦·½³ĢŹ½ HCl+NaOHØTNaCl+H2O
0.02610L”Į0.1000mol/L 0.025L”Įc£ØNaOH£©
Ōņc£ØNaOH£©=$\frac{0.02610L”Į0.1000mol/L}{0.025L}$=0.1044mol/L£¬
“š£ŗNaOHµÄÅضČĪŖ0.1044mol/L£®
µćĘĄ ±¾ĢāÖ÷ŅŖæ¼²éĮĖÖŠŗĶµĪ¶Ø²Ł×÷”¢Īó²ī·ÖĪöŅŌ¼°¼ĘĖć£¬ĢāÄæÄŃ¶Č²»“ó£¬Ąķ½āÖŠŗĶµĪ¶ØµÄŌĄķŹĒ½āĢā¹Ų¼ü£¬×¢ŅāĪó²ī·ÖĪöµÄ·½·Ø£¬ĪŖŅדķµć£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | CO2µÄĦ¶ūÖŹĮæĪŖ44g | |
| B£® | 1 mol N2µÄÖŹĮæŹĒ14g | |
| C£® | ±ź×¼×“æöĻĀ£¬1 mol CO2ĖłÕ¼µÄĢå»żŌ¼ŹĒ22.4L | |
| D£® | ½«40 g NaOHČÜÓŚ1 LĖ®ÖŠ£¬ĖłµĆČÜŅŗÖŠNaOHµÄĪļÖŹµÄĮæÅضČĪŖ1 mol/L |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
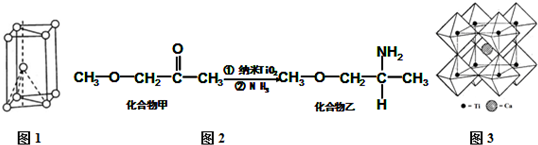
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 1.7gµÄOH-ÖŠŗ¬ÓŠµÄµē×ÓŹżĪŖNA | |
| B£® | ³£ĪĀ³£Ń¹ĻĀ£¬1.12LO2ÖŠĖłŗ¬ŃõŌ×ÓŹżĪŖNA | |
| C£® | 0.1molNa2O2ÖŠŗ¬O2-ŹżĪŖ0.2NA | |
| D£® | 1L1mol/LH2SO4ÖŠ£¬ŗ¬ÓŠ×ÜĄė×ÓŹżĪŖ3NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| Ń”Ļī | ²Ł×÷ | ĻÖĻó | ½įĀŪ |
| A | ijČÜŅŗ¼ÓČėK3[Fe£ØCN£©6]ČÜŅŗ | ŗŚÉ«³ĮµķÉś³É | Ö¤Ć÷ŌČÜŅŗÖŠ“ęŌŚFe2+ |
| B | ijČÜŅŗ¼ÓČėHNO3”¢BaCl2»ģŗĻŅŗ | ²śÉś°×É«³Įµķ | øĆČÜŅŗŅ»¶Øŗ¬ÓŠSO42- |
| C | ½«Ė®ÕōĘųĶعż×ĘČȵÄĢś·Ū | ·ŪÄ©ĪŽ±ä»Æ | ĢśÓėĖ®ÕōĘūøßĪĀĻĀ²»·“Ó¦ |
| D | ½«Ņ»Š”æéNa·ÅČėĪŽĖ®ŅŅ“¼ÖŠ | ²śÉśĘųÅŻ | NaÄÜÖĆ»»³ö“¼ōĒ»łÖŠµÄĒā |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 504mL | B£® | 168mL | C£® | 336mL | D£® | 224mL |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
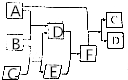 ÓŠA”¢B”¢C”¢D”¢E”¢FĮłÖÖĪ¢Į££¬ĘäÖŠA”«EĪåÖÖĪ¢Į£¾łÓÉĮ½ÖÖŌŖĖŲ×é³ÉĒŅ¾łŗ¬10øöµē×Ó£¬ĖüĆĒÓŠČēĶ¼ĖłŹ¾µÄ×Ŗ»Æ¹ŲĻµ£ŗ
ÓŠA”¢B”¢C”¢D”¢E”¢FĮłÖÖĪ¢Į££¬ĘäÖŠA”«EĪåÖÖĪ¢Į£¾łÓÉĮ½ÖÖŌŖĖŲ×é³ÉĒŅ¾łŗ¬10øöµē×Ó£¬ĖüĆĒÓŠČēĶ¼ĖłŹ¾µÄ×Ŗ»Æ¹ŲĻµ£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2016-2017ѧğ½Ī÷Ź”øßŅ»ÉĻµŚŅ»“ĪŌĀæ¼»Æѧ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ijµē½āÖŹČÜŅŗÖŠæÉÄÜ“óĮæ“ęŌŚH+”¢Mg2+”¢CO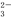 ”¢OH£”¢ Cl£µČĄė×ÓÖŠµÄ¼øÖÖ£¬ĻĀĮŠÓŠ¹ŲĖµĆ÷ÕżČ·µÄŹĒ
”¢OH£”¢ Cl£µČĄė×ÓÖŠµÄ¼øÖÖ£¬ĻĀĮŠÓŠ¹ŲĖµĆ÷ÕżČ·µÄŹĒ
A£®Ņ»¶ØƻӊMg2+”¢CO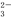 ”¢OH£ B£®Ņ»¶ØĪŽCl£
”¢OH£ B£®Ņ»¶ØĪŽCl£
C£®»¹ŠčŅŖ½ųŅ»²½Č·ČĻµÄĄė×ÓŹĒH+”¢Cl£ D£®ĪŽ·ØÅŠ¶ĻČÜŅŗÖŠŗ¬ÓŠŗĪÖÖŃōĄė×Ó
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2016-2017ѧğŗŚĮś½“óĒģŅ»ÖŠøßŅ»ÉĻ10ŌĀŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
Ąė×Ó·½³ĢŹ½BaCO3+2H+=CO2”ü+H2O+Ba2+ÖŠµÄH+ÄÜ“ś±ķµÄĪļÖŹŹĒ( )
¢ŁHCl ¢ŚH2SO4 ¢ŪHNO3 ¢ÜNaHSO4 ¢ŻCH3COOH
A£® ¢Ś¢Ü¢Ż B£®¢Ł¢Ü¢Ż C£®¢Ł¢Ż D£®¢Ł¢Ū
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com