�״���CH
3OH���Ͷ����ѣ�CH
3OCH
3������Ϊ21���͵�����ȼ�ϣ���CH
4��H
2OΪԭ���Ʊ������Ѻͼ״��Ĺ�ҵ�������£�
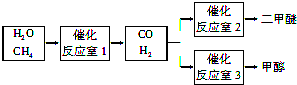
��1��д������Ӧ��1����һ�������½��еĻ�ѧ��Ӧ����ʽ��
��
��2����ѹǿΪ0.1MPa�����£���Ӧ��3���ݻ�ΪV L����a mol CO��2a mol H
2�ڴ��������·�Ӧ���ɼ״���CO��g��+2H
2��g��?CH
3OH��g����CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ1��ʾ����
��p
1
���������������=����p
2��
���������������������£���Ӧ��3������a mol CO��2a mol H2���ﵽ��ƽ��ʱ��CO��ת����
���������С�����䡱����
����p
1ѹǿ�£�100��ʱ����Ӧ��CH
3OH��g��?CO��g��+2H
2��g����ƽ�ⳣ��Ϊ
�����ú�a��V�Ĵ���ʽ��ʾ��
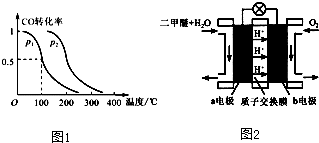
��3��ͼ2Ϊ��ɫ��Դ��������ȼ�ϵ�ء��Ĺ���ԭ��ʾ��ͼ��a�缫�ĵ缫��ӦʽΪ
��
��4��ˮú���ϳɶ����ѵ�������Ӧ���£�
��2H
2��g��+CO��g��?CH
3OH��g������H=-90.8kJ?mol
-1��2CH
3OH��g��?CH
3OCH
3��g��+H
2O��g������H=-23.5kJ?mol
-1��CO��g��+H
2O��g��?CO
2��g��+H
2��g������H=-41.3kJ?mol
-1��Ӧ��3H
2��g��+3CO��g��?CH
3OCH
3��g��+CO
2��g���ġ�H=
��








![]() ����õ�ط�Ӧ�������ӷ���ʽΪ________________________________________���״���_____________������_____________��Ӧ��
����õ�ط�Ӧ�������ӷ���ʽΪ________________________________________���״���_____________������_____________��Ӧ��