
| ŹµŃ鱹ŗÅ | ĘšŹ¼ÅضČc(HA) | ĘšŹ¼ÅضČc(NaOH) | ·“Ó¦ŗóČÜŅŗµÄpH |
| ¢Ł | 0.1 mol”¤L£1 | 0.1 mol”¤L£1 | 9 |
| ¢Ś | x | 0.2mol”¤L£1 | 7 |



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ŌŚpH£½1µÄČÜŅŗÖŠ£ŗK£«”¢Na£«”¢SO42ØC”¢HCO3£ |
| B£®ŌŚ0.1 mol”¤L£1 Na2CO3ČÜŅŗÖŠ£ŗAl3£«”¢K£«”¢NO3£”¢SO42ØC |
C£®ŌŚ0.1 mol”¤L£1 FeCl3ČÜŅŗÖŠ£ŗK£«”¢NH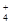 ”¢I£”¢SCN£ ”¢I£”¢SCN£ |
| D£®ŌŚc( H£«)/c(OH£)£½10£12µÄČÜŅŗÖŠ£ŗK£«”¢Na£«”¢ClO£”¢NO3£ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®HCO3- | B£®SO32- | C£®Cl£ | D£®Na£« |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®½«µČĪļÖŹµÄĮæÅØ¶Č”¢µČĢå»żµÄBa(OH)2ČÜŅŗŗĶNaHS04ČÜŅŗ»ģŗĻ£¬Éś³É°×É«³Įµķ£ŗBa2++SO42?+H++OH? =BaSO4”ż+H2O |
| B£®³£ĪĀĻĀ£¬µČĪļÖŹµÄĮæÅØ¶ČµÄČżÖÖČÜŅŗ¢Ł(NH4)2CO3£»¢ŚNH4Cl£»¢Ū(NH4)2Fe(SO4)2ÖŠc(NH4£«)£ŗ¢Ł£¼¢Ś£¼¢Ū |
| C£®¼ÓČė±½·ÓĻŌ×ĻÉ«µÄČÜŅŗÖŠ£ŗK£«”¢NH4£«”¢Cl£”¢I£ ĖÄÖÖĄė×Ó²»ÄÜ“óĮæ¹²“ę |
D£®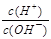 £½1”Į10£12µÄČÜŅŗ£ŗ K£«”¢AlO2£”¢CO32£”¢Na£«ĖÄÖÖĄė×ÓæÉŅŌ“óĮæ¹²“ę £½1”Į10£12µÄČÜŅŗ£ŗ K£«”¢AlO2£”¢CO32£”¢Na£«ĖÄÖÖĄė×ÓæÉŅŌ“óĮæ¹²“ę |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®pH£½1µÄČÜŅŗÖŠ£ŗFe3+”¢Cl£”¢NO3-”¢K+ |
| B£®µĪČė·ÓĢŖĻŌŗģÉ«µÄČÜŅŗÖŠ£ŗNa+”¢Mg2+”¢AlO2-”¢NO3- |
| C£®¼ÓČėĀĮ·Ū²śÉśH2µÄČÜŅŗÖŠ£ŗFe2+”¢Na+”¢SO42-”¢ClO£ |
| D£®c(SO32-)£½0.1 mol”¤L£1µÄČÜŅŗÖŠ£ŗNa+”¢Cl£”¢H+”¢Ca2+ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
| ŅõĄė×Ó | 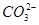 ”¢ ”¢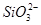 ”¢ ”¢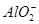 ”¢Cl£ ”¢Cl£ |
| ŃōĄė×Ó | Al3£«”¢Cu2£«”¢Mg2£«”¢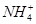 ”¢Na£« ”¢Na£« |
 Sn(OH)2
Sn(OH)2 Sn2£«£«2OH£”£
Sn2£«£«2OH£”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
A£®pH£½1µÄČÜŅŗÖŠ£ŗK£«”¢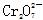 ”¢CH3CH2OH”¢ ”¢CH3CH2OH”¢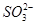 |
B£®ŗ¬ÓŠ“óĮæFe3£«µÄČÜŅŗÖŠ£ŗAl3£«”¢Cu2£«”¢Cl£”¢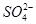 |
C£®¼ÓČėAlÄܷųöH2µÄČÜŅŗÖŠ£ŗK£«”¢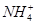 ”¢ ”¢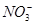 ”¢Cl£ ”¢Cl£ |
D£®Ė®µēĄėµÄc(H£«)£½10£12 mol/LµÄČÜŅŗÖŠ£ŗK£«”¢Ba2£«”¢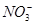 ”¢I£ ”¢I£ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®Ė®µÄĄė×Ó»ż³£ŹżKWÖ»ÓėĪĀ¶ČÓŠ¹Ų£¬µ«Ķā¼ÓĖį”¢¼ī”¢ŃĪŅ»¶Ø»įÓ°ĻģĖ®µÄµēĄė³Ģ¶Č |
| B£®Ksp²»½öÓėÄŃČܵē½āÖŹµÄŠŌÖŹŗĶĪĀ¶ČÓŠ¹Ų£¬»¹ÓėČÜŅŗÖŠĻą¹ŲĄė×ÓµÄÅضČÓŠ¹Ų |
C£®³£ĪĀĻĀ£¬ŌŚ0.10 mol”¤L£1µÄNH3”¤H2OČÜŅŗÖŠ¼ÓČėÉŁĮæNH4Cl¾§Ģ壬ÄÜŹ¹ČÜŅŗµÄpH¼õŠ”£¬c(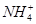 )/c(NH3”¤H2O)µÄÖµŌö“ó )/c(NH3”¤H2O)µÄÖµŌö“ó |
| D£®ŹŅĪĀĻĀ£¬CH3COOHµÄKW£½1.7”Į10£5£¬NH3”¤H2OµÄKb£½1.7”Į10£5£¬CH3COOHČÜŅŗÖŠµÄc(H£«)ÓėNH3”¤H2OÖŠµÄc(OH£)ĻąµČ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ŌŚŗ¬“óĮæFe3£«µÄČÜŅŗÖŠ£ŗNH4£«”¢Na£«”¢Cl£”¢SCN£ |
| B£®ŌŚĒæ¼īČÜŅŗÖŠ£ŗNa£«”¢K£«”¢AlO2£”¢CO32£ |
| C£®ŌŚc(H£«)£½10£13 mol”¤L£1µÄČÜŅŗÖŠ£ŗNH4£«”¢Al3£«”¢SO42£”¢NO3£ |
| D£®ŌŚpH£½1µÄČÜŅŗÖŠ£ŗK£«”¢I£”¢Cl£”¢NO3£ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com