
 ��������
��������
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
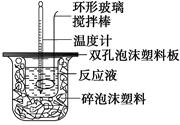
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
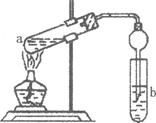

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
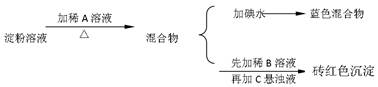
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ��һ�����飺ʵ�鷽����þ���Ͻ�
��һ�����飺ʵ�鷽����þ���Ͻ� �ⶨʣ���������
�ⶨʣ��������� ����ƽ����һ������þ���Ͻ��ĩ
����ƽ����һ������þ���Ͻ��ĩ ���������飺ʵ�鷽����þ���Ͻ�
���������飺ʵ�鷽����þ���Ͻ� �ⶨ������������
�ⶨ������������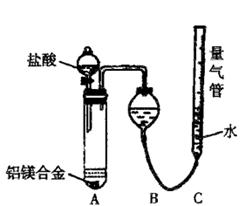

 �����յõ���������1��45g����úϽ���������������Ϊ ��
�����յõ���������1��45g����úϽ���������������Ϊ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 | 1 | 2 | 3 | 4 |
| ��������������Һ�����/mL | 25 | 25 | 25 | 25 |
| ���ɳ�����������/g | 0.29 | X | 0.87 | 0.87 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Cl2��ˮ��Һ���Ե��磬˵��Cl2�ǵ���� |
| B��������Fe(NO3)2������ˮ��Һ�У��μ�ϡH2SO4�ữ���ٵμ�KSCN��Һ���Ѫ��ɫ��˵��Fe(NO3)2�����Ѿ����� |
| C������۵⻯�ص���Һ�м�����ˮ����Һ��Ϊ��ɫ��˵��Cl2��������ǿ��I2 |
| D���ڵ��з�̪��Na2CO3��Һ�У�����BaCl2��Һ����Һ��ɫ,˵��BaCl2��Һ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����ȵ�ͭ˿��������ȼ�գ������ػ�ɫ���� |
| B����ˮ�м����������������ú��ϲ���ɫ��dz���²���ɫ��Ϊ��ɫ |
| C������ˮ�м���⻯����Һ����ˮ��Ϊ��ɫ |
| D��������Cl2��ȼ�ղ�����ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CO2��NaOH��Һ | B��NH3��ˮ | C��Cl2��NaOH��Һ | D��NO2��ˮ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com