
 ѧɜÓĆ0.1mol/L KOHČÜŅŗµĪ¶ØĪ“ÖŖÅØ¶ČµÄŃĪĖįČÜŅŗ£¬Ęä²Ł×÷æÉ·Ö½āĪŖČēĻĀ¼ø²½£ŗ
ѧɜÓĆ0.1mol/L KOHČÜŅŗµĪ¶ØĪ“ÖŖÅØ¶ČµÄŃĪĖįČÜŅŗ£¬Ęä²Ł×÷æÉ·Ö½āĪŖČēĻĀ¼ø²½£ŗ| µĪ¶Ø“ĪŹż | “ż²āČÜŅŗµÄĢå»ż/mL | ±ź×¼ČÜŅŗµÄĢå»ż/mL | |
| µĪ¶ØĒ°æĢ¶Č | µĪ¶ØŗóæĢ¶Č | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 2.00 | 21.99 |
| 3 | 25.00 | 0.20 | 20.20 |
·ÖĪö £Ø1£©øł¾ŻÖŠŗĶµĪ¶ØÓŠ¼ģĀ©”¢Ļ“µÓ”¢ČóĻ“”¢×°Ņŗ”¢Č”“ż²āŅŗ²¢¼ÓÖøŹ¾¼Į”¢µĪ¶ØµČ²Ł×÷£»¼īŠŌČÜŅŗŹ¢·ÅŌŚ¼īŹ½µĪ¶Ø¹ÜÖŠ£¬ĖįŠŌ»ņĒæŃõ»ÆŠŌČÜŅŗŹ¢·ÅŌŚĖįŹ½µĪ¶Ø¹ÜÖŠ£»
£Ø2£©µĪ¶Ø¹ÜŌŚ×°ŅŗŹ±£¬ĪŖĮĖ·ĄÖ¹ČÜŅŗ±»Ļ”ŹĶ£¬ŠčÓĆ“ż×°ŅŗČóĻ“£»
£Ø3£©øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©”ĮV£Ø±ź×¼£©}{V£Ø“ż²ā£©}$·ÖĪöĪó²ī£»
£Ø4£©ŹµŃéÖŠÓ¦æŲÖĘĮ÷ĖŁ²¢¹Ū²ģ׶ŠĪĘæÖŠŃÕÉ«±ä»Æ£»ČēČÜŅŗŃÕÉ«±ä»ÆĒŅ°ė·ÖÖÓÄŚ²»±äÉ«£¬æÉĖµĆ÷“ļµ½µĪ¶ØÖÕµć£»
£Ø5£©³ĘČ”Ņ»¶ØĮæµÄKOH¹ĢĢå£Øŗ¬ÉŁĮæNaOH£©£¬n£ØOH-£©Ę«“ó£¬ĻūŗÄĖįĘ«“ó£¬ÓÉc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©”ĮV£Ø±ź×¼£©}{V£Ø“ż²ā£©}$·ÖĪöĪó²ī£»
£Ø6£©ŃöŹÓ¹Ū²ģµĪ¶Ø¹ÜÖŠŅŗĆęæĢ¶Č£¬¶ĮŹżĘ«“󣬱ź×¼ŅŗµÄĢå»żĘ«“ó£¬øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©”ĮV£Ø±ź×¼£©}{V£Ø“ż²ā£©}$·ÖĪöĪó²ī£»
£Ø7£©øł¾Ż±ķÖŠŹż¾ŻĒó³ö±ź×¼ČÜŅŗµÄĢå»żĘ½¾łÖµ£¬ŌŁøł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©”ĮV£Ø±ź×¼£©}{V£Ø“ż²ā£©}$¼ĘĖć£®
½ā“š ½ā£ŗ£Ø1£©ÖŠŗĶµĪ¶Ø°“ÕÕ¼ģĀ©”¢Ļ“µÓ”¢ČóĻ“”¢×°Ņŗ”¢Č”“ż²āŅŗ²¢¼ÓÖøŹ¾¼Į”¢µĪ¶ØµČĖ³Šņ²Ł×÷£¬ŌņÕżČ·µÄĖ³ŠņĪŖBDCEAF£»¼īŠŌČÜŅŗŹ¢·ÅŌŚ¼īŹ½µĪ¶Ø¹ÜÖŠ£¬ĖłŅŌKOHČÜŅŗÓĆ¼īŹ½µĪ¶Ø£ØŅŅ£©¹ÜŹ¢·Å£¬ĖįŠŌ»ņĒæŃõ»ÆŠŌČÜŅŗŹ¢·ÅŌŚĖįŹ½µĪ¶Ø¹ÜÖŠ£¬ŌņøßĆĢĖį¼ŲČÜŅŗÓĆĖįŹ½µĪ¶Ø¹Ü£Ø¼×£©Ź¢·Å£»
¹Ź“š°øĪŖ£ŗBDCEAF£»ŅŅ£»¼×£»
£Ø2£©ÉĻŹö£ØB£©²Ł×÷µÄÄæµÄŹĒ·ĄÖ¹½«±ź×¼ŅŗĻ”ŹĶ£¬
¹Ź“š°øĪŖ£ŗ·ĄÖ¹½«±ź×¼ŅŗĻ”ŹĶ£»
£Ø3£©ÉĻŹö£ØA£©²Ł×÷Ö®Ē°£¬ČēĻČÓĆ“ż²āŅŗČóĻ“׶ŠĪĘ棬“ż²āŅŗµÄĪļÖŹµÄĮæĘ«“ó£¬ŌņĻūŗĵıź×¼ŅŗĢå»żĘ«“ó£¬øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©”ĮV£Ø±ź×¼£©}{V£Ø“ż²ā£©}$·ÖĪö£¬æÉÖŖc£Ø“ż²ā£©Ę«“ó£»
¹Ź“š°øĪŖ£ŗĘ«“ó£»
£Ø4£©µĪ¶ØŹ±£¬µĪ¶Ø¹ż³ĢÖŠ£¬ÓĆ×óŹÖæŲÖĘ¼īŹ½µĪ¶Ø¹ÜĻšĘ¤¹Ü²£Į§Ö铦£¬ÓŅŹÖŅ”¶Æ׶ŠĪĘ棬Į½ŃŪÓ¦øĆ×¢ŹÓ׶ŠĪĘæÄŚČÜŅŗµÄŃÕÉ«±ä»Æ£»µĪ¶ØŹ±£¬×¶ŠĪĘæÖŠČÜŅŗµÄŃÕÉ«ÓÉĪŽÉ«±äĒ³ŗģĒŅ±£³Ö30ĆėÄŚ²»ĶŹÉ«£¬æÉĖµĆ÷“ļµ½µĪ¶ØÖÕµć£»
¹Ź“š°øĪŖ£ŗ¼īŹ½µĪ¶Ø¹ÜĻšĘ¤¹Ü²£Į§Ö铦£»×¶ŠĪĘæÖŠČÜŅŗµÄŃÕÉ«±ä»Æ£»×¶ŠĪĘæÖŠČÜŅŗµÄŃÕÉ«ÓÉĪŽÉ«±äĒ³ŗģĒŅ±£³Ö30ĆėÄŚ²»ĶŹÉ«£»
£Ø5£©Čō³ĘČ”Ņ»¶ØĮæµÄKOH¹ĢĢå£Øŗ¬ÉŁĮæNaOH£©ÅäÖʱź×¼ČÜŅŗ²¢ÓĆĄ“µĪ¶ØÉĻŹöŃĪĖį£¬µČÖŹĮæµÄNaOH»ņKOH¹ĢĢåĄ“ÖŠŗĶĖįŹ±£¬NaOHĻūŗĵÄŃĪĖįµÄ¶ą£¬ĖłŅŌ»įŌģ³ÉV£Ø±ź£©Ę«Š”£¬øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©”ĮV£Ø±ź×¼£©}{V£Ø“ż²ā£©}$·ÖĪö£¬æÉÖŖc£Ø“ż²ā£©Ę«Š”£»
¹Ź“š°øĪŖ£ŗĘ«Š”£»
£Ø6£©ŃöŹÓ¹Ū²ģµĪ¶Ø¹ÜÖŠŅŗĆęæĢ¶Č£¬¶ĮŹżĘ«“󣬱ź×¼ŅŗµÄĢå»żĘ«“ó£¬øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©”ĮV£Ø±ź×¼£©}{V£Ø“ż²ā£©}$·ÖĪö£¬æÉÖŖc£Ø“ż²ā£©Ę«“ó£»
¹Ź“š°øĪŖ£ŗĘ«“ó£»
£Ø7£©±ź×¼ČÜŅŗµÄĢå»żĘ½¾łÖµĪŖ$\frac{£Ø21.03-1.02£©+£Ø21.99-2.00£©+£Ø20.20-0.20£©}{3}$=20.00mL£¬c£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©”ĮV£Ø±ź×¼£©}{V£Ø“ż²ā£©}$=$\frac{0.1mol/L”Į0.020L}{0.025L}$=0.08mol/L£»
¹Ź“š°øĪŖ£ŗ0.08mol/L£®
µćĘĄ ±¾Ģāæ¼²éĖį¼īÖŠŗĶµĪ¶ØŹµŃ飬ĢāÄæÄŃ¶Č²»“ó£¬×¢Ņā°ŃĪÕŹµŃéµÄ²½Öč”¢·½·ØŅŌ¼°×¢ŅāŹĀĻī£¬ŌŚĪó²ī·ÖĪöŹ±øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©”ĮV£Ø±ź×¼£©}{V£Ø“ż²ā£©}$·ÖĪö¼“æÉ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ca£ØHCO2£©2ČÜŅŗÖŠ¼ÓČė¹żĮæNaOHČÜŅŗCa2++HCO${\;}_{3}^{-}$+OH-ØTCaCO3”ż+H2O | |
| B£® | ĖįŠŌĢõ¼žĻĀKIO3ČÜŅŗÓėKIČÜŅŗ·“Ó¦IO${\;}_{3}^{-}$+5I-+3H2OØT3I2+6OH- | |
| C£® | AlCl3ČÜŅŗÖŠ¼ÓČėÉŁĮæNa””2Al3++6Na+6H2OØT2Al£ØOH£©3”ż+6Na++3H2”ü | |
| D£® | FeBr2ČÜŅŗÖŠĶØČėÉŁĮæCl2””2Fe2++4Br+3Cl2”ś2Fe3++2Br2+6Cl- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
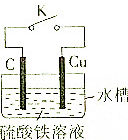 ijĶ¬Ń§ĪŖĮĖĢ½¾æŌµē³ŲŌĄķ£¬Éč¼ĘČēĶ¼ĖłŹ¾×°ÖĆ£ŗ
ijĶ¬Ń§ĪŖĮĖĢ½¾æŌµē³ŲŌĄķ£¬Éč¼ĘČēĶ¼ĖłŹ¾×°ÖĆ£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ijæĪĶāŠĖȤŠ”×éÓū²ā¶ØijNaOHČÜŅŗµÄÅØ¶Č£¬Ęä²Ł×÷²½ÖčČēĻĀ£ŗ
ijæĪĶāŠĖȤŠ”×éÓū²ā¶ØijNaOHČÜŅŗµÄÅØ¶Č£¬Ęä²Ł×÷²½ÖčČēĻĀ£ŗ| µĪ¶Ø“ĪŹż | “ż²āŅŗĢå»ż£ØmL£© | ±ź×¼ŃĪĖįĢå»ż£ØmL£© | |
| µĪ¶ØĒ°¶ĮŹż£ØmL£© | µĪ¶Øŗó¶ĮŹż£ØmL£© | ||
| µŚŅ»“Ī | 25.00 | 0.50 | 20.40 |
| µŚ¶ž“Ī | 25.00 | 4.00 | 24.10 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
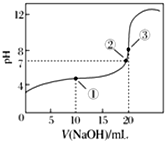 ³£ĪĀĻĀ£¬ÓĆ0.100 0mol•L-1 NaOHČÜŅŗµĪ¶Ø20.00mL 0.100 0mol•L-1 CH3COOHČÜŅŗµĪ¶ØĒśĻßČēĶ¼ĖłŹ¾£®ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©
³£ĪĀĻĀ£¬ÓĆ0.100 0mol•L-1 NaOHČÜŅŗµĪ¶Ø20.00mL 0.100 0mol•L-1 CH3COOHČÜŅŗµĪ¶ØĒśĻßČēĶ¼ĖłŹ¾£®ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©| A£® | µć¢ŁĖłŹ¾ČÜŅŗÖŠ£ŗc£ØNa+£©=c£ØCH3COOH£©+c£ØCH3COO-£© | |
| B£® | µć¢ŚĖłŹ¾ČÜŅŗÖŠ£ŗc£ØNa+£©=c£ØCH3COOH£©+c£ØCH3COO-£© | |
| C£® | µć¢ŪĖłŹ¾ČÜŅŗÖŠ£ŗc£ØNa+£©£¾c£ØOH-£©£¾c£ØCH3COO-£©£¾c£ØH+£© | |
| D£® | µĪ¶Ø¹ż³ĢÖŠæÉÄܳöĻÖ£ŗc£ØCH3COOH£©£¾c£ØCH3COO-£©£¾c£ØH+£©£¾c£ØNa+£©£¾c£ØOH-£© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
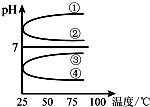 A”¢B”¢C”¢D”¢EĪåÖÖČÜŅŗ·Ö±šŹĒNaOH”¢NH3•H2O”¢CH3COOH”¢HCl”¢NH4HSO4ÖŠµÄŅ»ÖÖ£®³£ĪĀĻĀ½ųŠŠĻĀĮŠŹµŃé£ŗ
A”¢B”¢C”¢D”¢EĪåÖÖČÜŅŗ·Ö±šŹĒNaOH”¢NH3•H2O”¢CH3COOH”¢HCl”¢NH4HSO4ÖŠµÄŅ»ÖÖ£®³£ĪĀĻĀ½ųŠŠĻĀĮŠŹµŃé£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
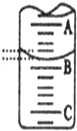 ij»ÆѧŹµŃ銔×éĻėŅŖĮĖ½āŹŠ³”ÉĻĖłŹŪŹ³ÓĆ°×“×£ØÖ÷ŅŖŹĒ“×ĖįµÄĖ®ČÜŅŗ£©µÄ×¼Č·ÅØ¶Č£¬ĻÖ“ÓŹŠ³”ÉĻĀņĄ“Ņ»ĘæÄ³Ę·ÅĘŹ³ÓĆ°×“×£¬ÓĆŹµŃéŹŅ±ź×¼NaOHČÜŅŗ¶ŌĘä½ųŠŠµĪ¶Ø£®ĻĀ±ķŹĒ4ÖÖ³£¼ūÖøŹ¾¼ĮµÄ±äÉ«·¶Ī§£ŗ
ij»ÆѧŹµŃ銔×éĻėŅŖĮĖ½āŹŠ³”ÉĻĖłŹŪŹ³ÓĆ°×“×£ØÖ÷ŅŖŹĒ“×ĖįµÄĖ®ČÜŅŗ£©µÄ×¼Č·ÅØ¶Č£¬ĻÖ“ÓŹŠ³”ÉĻĀņĄ“Ņ»ĘæÄ³Ę·ÅĘŹ³ÓĆ°×“×£¬ÓĆŹµŃéŹŅ±ź×¼NaOHČÜŅŗ¶ŌĘä½ųŠŠµĪ¶Ø£®ĻĀ±ķŹĒ4ÖÖ³£¼ūÖøŹ¾¼ĮµÄ±äÉ«·¶Ī§£ŗ| ÖøŹ¾¼Į | ¼×»ł³Č | ¼×»łŗģ | ŹÆČļ | ·ÓĢŖ |
| ±äÉ«·¶Ī§£ØpH£© | 3.1-4.4 | 4.4-6.2 | 5.0-8.0 | 8.2-10.0 |
| ŹµŃé“ĪŹż | µŚŅ»“Ī | µŚ¶ž“Ī | µŚČż“Ī |
| ĻūŗÄNaOHČÜŅŗĢå»ż/mL | 21.02 | 20.32 | 20.28 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | V£ØNaOH£©=0Ź±£¬c£ØNa+£©£¾c£ØH+£©£¾c£ØHC2O4- £©£¾c£ØC2O42-£©£¾c£ØOH-£© | |
| B£® | V£ØNaOH£©=5 mLŹ±£¬c£ØNa+£©ØTc£ØC2O42-£©+c£ØHC2O4- £©+c£ØH2C2O4£© | |
| C£® | V£ØNaOH£©=10 mLŹ±£¬c£ØNa+£©ØTc£ØHC2O4-£©+c£ØC2O42-£© | |
| D£® | V£ØNaOH£©£¾10 mLŹ±£¬c£ØNa+£©£¾c£ØC2O42-£©£¾c£ØHC2O4- £© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com