
��ͼ��������KAl(SO4)2•12H2O����ȡ������������صIJ�����������ͼ��ͼ����ȥ�˳������ϴ�Ӳ�����������ͼ��Բ�����������ʵ����Լ����ƣ��ڷ������������ʵ��ķ��뷽�������ش��й����⡣
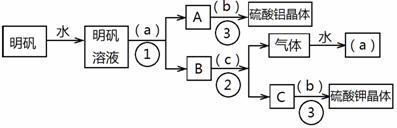
��1���Լ�(a)��________________��(b)��______________��(c)��____________��
��2�����뷽������________________������____________________;������������Ҫ�õ��IJ���������_______________________________��
��3����������������������ģ���������158g������Ħ������474g/mol���������Ƶ����������塲Al2(SO4)3•18H2O��(Ħ������666g/mol) _____g�����ٿ����Ʊ�K2SO4(Ħ������174g/mol)_______g��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣���ͼ��������KAl(SO4)2•12H2O����ȡ������������صIJ�����������ͼ��ͼ����ȥ�˳������ϴ�Ӳ�����������ͼ��Բ�����������ʵ����Լ����ƣ��ڷ������������ʵ��ķ��뷽�������ش��й����⡣
��1���Լ�(a)��________________��(b)��______________��(c)��____________��
��2�����뷽������________________������____________________;������������Ҫ�õ��IJ���������_______________________________��
��3����������������������ģ���������158g������Ħ������474g/mol���������Ƶ����������塲Al2(SO4)3•18H2O��(Ħ������666g/mol) _____g�����ٿ����Ʊ�K2SO4(Ħ������174g/mol)_______g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ2010-2011ѧ�����10���¿����⣨��ѧ�� ���ͣ������
��12�֣���ͼ��������KAl(SO4)2•12H2O����ȡ������������صIJ�����������ͼ��ͼ����ȥ�˳������ϴ�Ӳ�����������ͼ��Բ�����������ʵ����Լ����ƣ��ڷ������������ʵ��ķ��뷽�������ش��й����⡣

��1���Լ�(a)��________________��(b)��______________��(c)��____________��
��2�����뷽������________________������____________________;������������Ҫ�õ��IJ���������_______________________________��
��3����������������������ģ���������158g������Ħ������474g/mol���������Ƶ����������塲Al2(SO4)3•18H2O��(Ħ������666g/mol) _____g�����ٿ����Ʊ�K2SO4(Ħ������174g/mol)_______g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��������KAl(SO4)2??12H2O����ȡ������������صIJ�����������ͼ��ͼ����ȥ�˳������ϴ�Ӳ�����������ͼ��Բ�����������ʵ����Լ����ƣ��ڷ������������ʵ��ķ��뷽�������ش��й����⡣
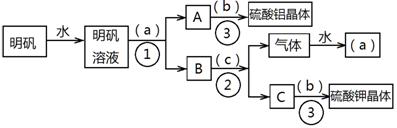
��1���Լ�(a)��________________��(b)��______________��(c)��____________��
��2�����뷽������________________������____________________;������������Ҫ�õ��IJ���������_______________________________��
��3����������������������ģ���������158g������Ħ������474g/mol���������Ƶ����������塲Al2(SO4)3??18H2O��(Ħ������666g/mol) _____g�����ٿ����Ʊ�K2SO4(Ħ������174g/mol)_______g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʦ����2011�����10���¿� ���ͣ������
��ͼ��������KAl��SO4��2•12H2O����ȡ������������صIJ�����������ͼ��ͼ����ȥ�˳������ϴ�Ӳ�����������ͼ��Բ�����������ʵ����Լ����ƣ��ڷ������������ʵ��ķ��뷽�������ش��й����⡣
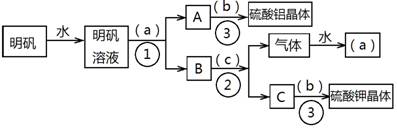
��1���Լ���a����________________����b����______________����c����____________��
��2�����뷽������________________������____________________;������������Ҫ�õ��IJ���������_______________________________��
��3����������������������ģ���������158g������Ħ������474g/mol���������Ƶ����������塲Al2��SO4��3•18H2O����Ħ������666g/mol�� _____g�����ٿ����Ʊ�K2SO4��Ħ������174g/mol��_______g��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com