
| ���� | ������ƽ | С�ձ� | ����ǯ | ������ | ҩ�� | ��Ͳ |
| ���� |  |  |  |  |  | |
| ��� | a | b | c | d | e | f |
| ʵ�� ���� | ��ʼ�¶�t1/�� | ��ֹ�¶� t2/�� | �¶Ȳ�ƽ��ֵ ��t2-t1��/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 30.1 | |
| 2 | 27.0 | 27.4 | 27.2 | 33.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.8 | |
| 4 | 26.4 | 26.2 | 26.3 | 30.4 | |
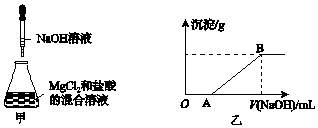
���� ��1������������Һ���ѡ����Ҫ���ʹ�������ƿ������m=CVM������Ҫ���ʵ�������
��2������������������ƽ��ҩ�ף��������ƾ��и�ʴ�ԣ�Ӧ����С�ձ��г�����
��1�������к�����Ҫ������Ӧǰ����¶ȣ���Ӧ��ų��������ʱ�¶ȣ�Ϊ��֤����ַ�Ӧ����Ҫ�û��β�����������裻
��2��������ͼ�����ݼ��㷴Ӧ�¶ȱ仯ƽ��ֵ��ע�����ϴ��ֵӦ������
�������кͷ�Ӧ��������1molҺ��ˮ�ų�����������
��A��ʵ��װ�ñ��¡�����Ч�����õ�����ƫС��
B����ȡNaOH��Һ�����ʱ���Ӷ�����������ȡ�������������ƫ��Ӧ�ų�����������
C����NaOH��Һ����С�ձ��У����ּܷ��ε��룬����ᵼ������ɢʧ��
D�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�Ӳⶨ������Һ�¶ȣ��������ʼ�¶�ƫ�ߣ�
��� �⣺�� ��1����ʵ���д�ԼҪʹ��0.50 mol/L�� 470 mL NaOH��Һ����������ƿ�����������ӽ�����500ml������Ӧ��ѡ��500ml����ƿ�����ƣ���Һ�о�һ�ԣ�������Ũ����ȣ�����������Ҫ����NaOH�����������m��NaOH��=��0�� 5L��0.50 mol/L����40g/mol=10.0g��
�ʴ�Ϊ��10.0��
��2������NaOH��������Ҫ����������ҩ��ȡҩƷ������NaOH�и�ʴ�ԣ��������ձ���ʹ��������ƽ���г�����
��ѡ��a��b��e��
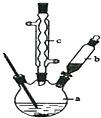
�ⶨ�к��ȣ���1��ʵ�����ϱ����ձ�����С�����ձ�������ĭ���ϡ���ĭ���ϰ塢��ͷ�ιܡ���Ͳ���ᡢNaOH��Һ����ȱ�ٵ�ʵ�鲣����Ʒ�ǻ��β�����������¶ȼƣ�
�ʴ�Ϊ�����β�����������¶ȼƣ�
��2���������Ĵ�ʵ���з�Ӧ���¶ȱ仯�ֱ��ǣ�5��6.1��3.9��4.1���ɼ���һ�Ρ��ڶ��ε����̫��ƫ����ʵֵ����ȥ��ƽ���¶��ǣ�3.9+4.1����2=4.0��
�ʴ�Ϊ��4.0��
�����������Ƕ�Ԫ�ᣬNaOH��һԪ����Ը��ݸ�����Ũ������Һ�������֪���������Ӧ�ų�������Ӧ�ð��ռ������㣬���Ը÷�Ӧ���к��ȡ�H=-$\frac{4.18��80��4.0��0.001KJ}{0.5��0.05mol}$=-53.5KJ/mol��
�ʴ�Ϊ��-53.5KJ/mol��
������ʵ����ֵ�����57.3 kJ•mol-1��ƫ�����ƫ���ԭ������ǣ�
A��ʵ��װ�ñ��¡�����Ч���ʹ����ɢʧ����Ӧ�ų�����ƫС����Aѡ��
B����ȡNaOH��Һ�����ʱ���Ӷ������������ʵ�������Ӧ�ų�����������B��ѡ��
C���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У�ʱ�����������ɢʧ�Ķ࣬��Ӧ�ų�����ƫС����Cѡ��
D�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶ȣ�ʹ��Ӧ��ʼ���¶�ƫ�ߣ��¶Ȳ�ƫС����Ӧ��ƫ�٣���Dѡ��
��ѡ��ACD��
���� ���⿼�����к��Ȳⶨʵ�飬ע�������к��ȵĸ�������Ȼ�ѧ����ʽ����д�����Լ��ⶨ��Ӧ�ȵ�����ǽ���ؼ�����Ŀ�ѶȲ���


 �γ̴����Ծ�����100��ϵ�д�
�γ̴����Ծ�����100��ϵ�д� �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д� ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���³�ѹ�£�18.0 g NH4+��������������Ϊ10 NA | |
| B�� | �����£�8 g���麬�й��ۼ���Ϊ2NA | |
| C�� | �����£�2.7 g��ƬͶ��������Ũ�����У���ʧȥ�ĵ�����Ϊ0.3NA | |
| D�� | �����£�1 mol/L �� Na2SO4��Һ�к���Na+����Ϊ2NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
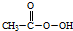 ���������ᣩ+H2O
���������ᣩ+H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ζ�����ˮϴ��δ�ñ���Һ��ϴ��װ�����Һ | |
| B�� | ����ȡ����Һ�ĵζ���δ�ô���Һ��ϴ | |
| C�� | ��ҡ����ƿ�Ĺ����в���������һС������Һ | |
| D�� | ��Һ����ʱ���ζ�ǰ���ӣ��ζ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
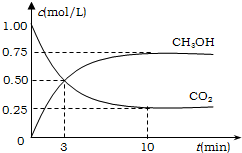 ��ҵ����һ�ַ�����Ч�ؿ�������CO2������CO2������ȼ�ϼ״���Ϊ̽����Ӧԭ������������ʵ�飬�����Ϊ1L�ĺ����ܱ������У�����1molCO2��3molH2��һ�������·�����Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g������H=-49.0kJ/mol�����CO2��CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ��
��ҵ����һ�ַ�����Ч�ؿ�������CO2������CO2������ȼ�ϼ״���Ϊ̽����Ӧԭ������������ʵ�飬�����Ϊ1L�ĺ����ܱ������У�����1molCO2��3molH2��һ�������·�����Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g������H=-49.0kJ/mol�����CO2��CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
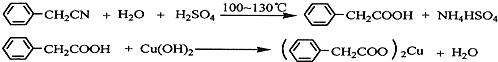
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
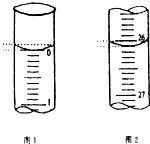 ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ�������ָʾ��������д���пհף�
ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ�������ָʾ��������д���пհף�| �ζ����� | ��������������Һ�����/mL | 0.1000mol•L-1��������/mL | ||
| �ζ�ǰ�̶� | �ζ���̶� | ��Һ���/mL | ||
| ��һ�� | 25.00 | 0.00 | 26.11 | 26.11 |
| �ڶ��� | 25.00 | 1.56 | 30.30 | 28.74 |
| ������ | 25.00 | 0.22 | 26.31 | 26.09 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com