
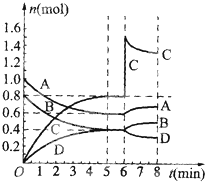
 �п��淴ӦA��g��+B��g��?xC��g��+D��g����ij�¶�ʱ���ڹ̶��ݻ�Ϊ2L���ܱ������ڳ���һ������A��B���������¶Ȳ��䣮A��B��C��D���ʵ�����ʱ��ı仯��ͼ��ʾ��
�п��淴ӦA��g��+B��g��?xC��g��+D��g����ij�¶�ʱ���ڹ̶��ݻ�Ϊ2L���ܱ������ڳ���һ������A��B���������¶Ȳ��䣮A��B��C��D���ʵ�����ʱ��ı仯��ͼ��ʾ������ ��1�����ݸ������ʵ����ʵ����仯��֮�ȵ��ڷ���ʽ��ϵ��֮����ȷ��x���ﵽ��ƽ��״̬ʱ������ֵ����ʵ�������ʱ��ı仯���仯��
��2�����ݷ�Ӧ����v=$\frac{��c}{��t}$�����м��㼴�ɣ�
��3������ͼʾ������C�����ʵ���Ѹ�����ӣ�����ƽ�������ƶ���A��B�����ʵ������ӣ�D�����ʵ�����С��
��4����Ӧһ��ʼ�ͼ����˺��ʵĴ�������ӿ췴Ӧ���ʣ����Ƿ�Ӧ��ƽ��״̬����ı䣮
��� �⣺��1���������ʵ����ʵ����仯��֮�ȵ��ڷ���ʽ��ϵ��֮�ȣ���0-5minʱ����ڣ����ʵı仯��֮��ΪA��B��C��D=0.4��0.4��0.8��0.4=1��1��2��1����x=2��
�ﵽ��ƽ��״̬ʱ������ֵ����ʵ�������ʱ��ı仯���仯������5-6min��ƽ��״̬���ʴ�Ϊ��2��5-6��
��2����Ӧ����v=$\frac{��c}{��t}$=$\frac{\frac{1.0mol-0.6mol}{2L}}{5min}$0.04mol/��L•min�����ʴ�Ϊ��0.04mol/��L•min����
��3������ͼʾ������6minʱC�����ʵ���Ѹ�����ӣ�����ƽ�������ƶ���A��B�����ʵ������ӣ�D�����ʵ�����С���ʴ�Ϊ������c��
��4����Ӧһ��ʼ�ͼ����˺��ʵĴ�������ӿ췴Ӧ���ʣ����̴ﵽƽ�����õ�ʱ�䣬���Ƿ�Ӧ��ƽ��״̬����ı䣬����ͼ��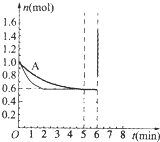 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
���� ���⿼��ѧ����ѧ��Ӧ���ʵļ��㡢��ѧƽ����ж��Լ�ͼ��֪ʶ��Ӧ�õȣ��Ѷ��еȣ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ˮ��c��Cl-����2[c��Fe2+��+c��Cu2+��]�����ܴ���Fe3+ | |
| B�� | ���ˮ�м��������ᣬ�μ�KSCN��Һ����Һ��죬˵����ˮ�к���Fe3+ | |
| C�� | ȡһ�����ķ�ˮ��Ʒ�����ⶨ������n��Cu2+��=0.01mol��n��Fe2+��+n��Fe3+��=0.01mol�����ˮ����0mol��n��Cl-����0.05mol | |
| D�� | ȡ10 mL��ˮ��ͨ������Cl2 22.4mLǡ����ȫ��Ӧ��Ȼ�����pH��ʹ��Һ�е���Ԫ��ȫ��ת��ΪFe��OH��3�����������ˡ�ϴ�ӡ����գ���ȴ����ص�0.16g�����ˮ��c��Fe3+��=0.01 mol•L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ϡ���� | B�� | ϡ���� | C�� | NaOH��Һ | D�� | ����ͭ��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
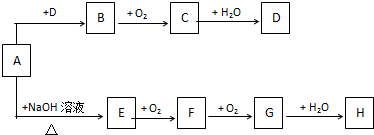
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | CH3-CH3 | B�� | CH3COOH | C�� | CH3COOCH3 | D�� | CH3COCH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������ӵ���ˮ�� | B�� | NaAlO2��Һ�м����������� | ||
| C�� | ��Ͷ�백ˮ�� | D�� | NaOH��������AlCl3��Һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢ� | B�� | �ۢڢ� | C�� | �ڢ٢� | D�� | �ڢۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
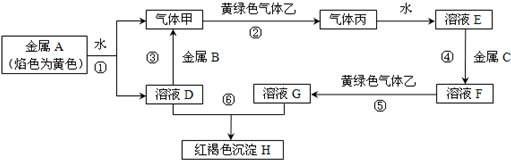
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �� �����۱��� | B�� | �� ����ɫ��ӦΪ��ɫ | ||
| C�� | ��Ȫˮ�к��иơ�þ�ȿ����� | D�� | ��ͬ���������к��ס����� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com