

�����
(1)A��B��Ӧ��������ʵĻ�ѧʽ�� A ��B ��
(2)A��B����Ӧ�����ӷ���ʽ��
A�� �� B�� ��
(3)ÿ����
(4)��ȥ�Ⱥ�ķ�ˮ��������Ũ��ԼΪ���٣�
�������÷�ˮ��������������Ҫ��һ�dz�ȥCl2�����ǵ�Ϊ���ԡ���ΪCl2��![]() ��ˮ��Ӧʱ������H+�����Գ�ȥH+Ӧ�ڸ÷�Ӧ֮����С�
��ˮ��Ӧʱ������H+�����Գ�ȥH+Ӧ�ڸ÷�Ӧ֮����С�
ÿ![]() Na2SO4+2HCl��֪������Na2SO3������HCl�����ʵ����ֱ�Ϊ
Na2SO4+2HCl��֪������Na2SO3������HCl�����ʵ����ֱ�Ϊ
n(Na2SO3)=12mol
n(HCl)=2��12mol=24mol
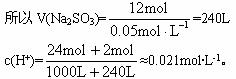
�𰸣�(1)Na2SO3 NaOH
(2)Cl2+![]() +H2O
+H2O![]()
![]() +2H++2Cl- H++OH-
+2H++2Cl- H++OH-![]() H2O
H2O
(3)



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�022

�����
��1��A��B��Ӧ��������ʵĻ�ѧʽ��A________��B________��
��2��A��B����Ӧ�����ӷ���ʽ��
A��________________��
B��________________��
��3��ÿ����1m3�ĸ÷�ˮ�������0.05mol��L-1��Na2SO3��Һ������Ƕ��٣�
��4����ȥ�Ⱥ�ķ�ˮ�а�����Ũ��ԼΪ���٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�038
ij�����ų��ķ�ˮ�ﺬ ������̬�Ⱥ�
������̬�Ⱥ� �������ӣ�Ϊ�˳�ȥ��ˮ�е�����̬�ȣ�����ʹ��ˮ��Ϊ���ԣ������������ͼ��ʾ�������ڷ�ˮ�ų��ܵ�A��B���ֱ�ע��һ�������ķ��ռ���Һ������������Һ��
�������ӣ�Ϊ�˳�ȥ��ˮ�е�����̬�ȣ�����ʹ��ˮ��Ϊ���ԣ������������ͼ��ʾ�������ڷ�ˮ�ų��ܵ�A��B���ֱ�ע��һ�������ķ��ռ���Һ������������Һ��
��д����(1)A��B��Ӧ��������ʵĻ�ѧʽ��A_____________��B____________________________________________________________��
(2)A��B����Ӧ�����ӷ���ʽ��A��_______________________________��
B��___________________________________________________________��
(3)ÿ���� �ĸ÷�ˮ�������
�ĸ÷�ˮ������� ��
�� ��Һ������Ƕ���?
��Һ������Ƕ���?
(4)��ȥ�Ⱥ�ķ�ˮ��������Ũ��ԼΪ����?
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�����
��1��A��B��Ӧ��������ʵĻ�ѧʽ��
A_________��B_________��
��2��A��B����Ӧ�����ӷ���ʽ
A��___________________________��
B��___________________________��
��3��ÿ����1 m3�ĸ÷�ˮ�������0.05 mol��L-1��Na2SO3��Һ������Ƕ��٣�
��4����ȥ�Ⱥ�ķ�ˮ��������Ũ��ԼΪ���٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�����
(1)A��B��Ӧ��������ʵĻ�ѧʽ��A______________��B______________��
(2)A��B����Ӧ�����ӷ���ʽ��
A��___________________________________________________________��
B��___________________________________________________________��
(3)ÿ����
(4)��ȥ�Ⱥ�ķ�ˮ��������Ũ��ԼΪ���٣�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com