
������������:��ͭ�����Ǣ�CuO��NaHSO4 ��Ba��OH��2�������������ɱ��ᵨ����ˮ
��1���������ʷ����в����ڵ���ʵ��� ������ţ�������ˮ��Һ�еĵ��뷽��ʽΪ ��
��2��̼��������������մ����������ʳ�����ơ���һ�������£���������������������̼������ʳ�ε������� ������ţ���
��3������������������������ˮ��Һ�з�����Ӧ,�����ӷ���ʽΪ:H++OH-=H2O,��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��4����� ��Һ�л����μӢ� ��Һ�������Һ�պó�����ʱ�����ӷ���ʽΪ��_______��
��5������������������������һ������������ÿ�����һ�������ʡ���ʽ̼��ͭ��Cu2��OH��2 CO3]����д���÷�Ӧ�Ļ�ѧ��Ӧ����ʽ________________ ��
��1�� �٢ڢޢߢ� NaHSO4=Na++H++SO42-
��2���ܢݢߢ��
��3��Ba��OH��2+2HCl=BaCl2+2H2O
��4��2OH-+SO42-+Ba2++2H��=2H2O+BaSO4��
��5��2Cu+O2+CO2+H2O=Cu2��OH��2 CO3
���������������1���������ָ��ˮ��Һ�������״̬�µ���Ļ���������ʵķ���Ƕ����������ᡢ��Ρ����������ˮ�ȣ�����ֻ�Тۢܢݢ��ʱ����ʣ�NaHSO4����ʽ���Σ�����������ǿ�ᣬ������ˮ��Һ�еĵ��뷽��ʽΪ��NaHSO4=Na++H++SO42-��
��2������̼������ʳ��ʵ��������Cl-��CO32-����CO32-��Ӧ�������Тܢݢߢ�ᣬ�ܢ߲������壬�ݢ������������ᱻ���ա�
��3��H++OH-=H2O�����ӷ���ʽ��ʾǿ����ǿ�����������εķ�Ӧ��������10��������ֻ�Тݢ߷��ϣ��仯ѧ����ʽΪ��Ba��OH��2+2HCl=BaCl2+2H2O��
��4����Һǡ�ó�����ʱ˵��H+��OH-ǡ����ȫ��Ӧ�����������������H+��OH-�ĸ����ȣ��ɵ� 2 OH-+SO42-+Ba2++2H��=2H2O+BaSO4��
��5��������ĿҪ��������������һ������������ÿ�����һ�������ʡ���ʽ̼��ͭ��Cu2��OH��2 CO3]�����������ʵĻ�ѧʽ���ѵó����������ʷֱ���ͭ��������ˮ��������̼��
���㣺�������ʵ��жϡ����Ӽ������ӷ���ʽ�����塢���ӷ���ʽ����д����ѧʽ�ķ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�������Թ�ҵ��ˮ�к���һ������Na����Al3����Fe3����Cu2����Cl�����ó�������ͼ��ʾ�Ĺ�������ͼ�����ó���������������ᡢ���ҵ�����еķ���м���ӷ�ˮ�����������Ȼ�������������NaCl����ͽ���ͭ�������˺ܺõ���ᾭ��Ч�档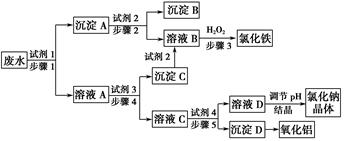
����д���пհף�
(1)ͼ���Լ�1��________���Լ�2��________��
(2)����1�Ͳ���2���õ��IJ���������________��
(3)����1��Ӧ�����ӷ���ʽΪ__________________________________��
(4)����3��Ӧ�����ӷ���ʽΪ__________________________________��
(5)�ӽ�ԼҩƷ�ͻ������濼�ǣ�����5��������Ӧ�����ӷ���ʽӦΪ____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������A��B����ѧ���������ʣ����������ӿɴ��±���ѡ��
| ������ | K����Na����Fe2����Ba2����NH4+ |
| ������ | OH����NO3����I����HCO3����AlO2����HSO4�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)�����Խ����У���MnSO4��Һ��μ�(NH4)2S2O8(���������)��Һ�ᷢ���������ӷ�Ӧ(δ��ƽ)��
Mn2����S2O82����H2O��MnO4����SO42����H����
�ٸ÷�Ӧ�����ڼ���Mn2���Ĵ��ڣ�������������___________________________________��
������Ӧ����0.1 mol��ԭ���μӷ�Ӧ����ת�Ƶ�����Ϊ________NA�����������������ʵ���______________mol��
��д���÷�Ӧ�����ӷ���ʽ_________________________________��
(2)����CuSO4��Һ��ͨ���������ɺ�ɫ����CuS�����ӷ���ʽΪ___________________________________��
����FeCl3��Һ�м�������ĵ⻯����Һ�����ӷ���ʽΪ_____________��
(3)�ڼ��Խ����У�H2O2�н�ǿ�Ļ�ԭ�ԣ�����Ag2O��Ӧ���÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ___________________��
(4)Ϊ�ⶨ�����еij���(O3)��������0�桢1.01��105 Pa�Ŀ���V L����ͨ������KI��Һ��ʹ������ȫ��Ӧ��Ȼ��������Һ��a mL c mol��L��1��Na2S2O3��Һ���еζ���ǡ�ôﵽ�յ㡣��֪��2Na2S2O3��I2=Na2S4O6��2NaI��
�ٸõζ������п�ѡ���ָʾ��Ϊ________��
��O3��KI��Һ��Ӧ�������ֵ��ʣ���Ӧ�Ļ�ѧ����ʽΪ___________________________��
�ۿ����г������������Ϊ________(�ú���a��c��V������ĸ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������ʣ���Һ�����ڰ�ˮ��������ܴ�������ᱵ�����������Ȼ��ƾ��壻�������̼�������泥���ƾ���Һ��
��1�����ڵ���ʵ���_____________________________________________________________��
��2���ܵ������_________________________________________________________________��
��3������ǿ����ʵ���___________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��С�մ���ij������θ������θҩ����Ҫ�ɷ֣���д���÷�Ӧ�����ӷ���ʽ�� ��
����ͨ��ˮ�п��Ƶð�ˮ����ˮ��ʹ��ɫ�ķ�̪��졣��д��ʵ������ȡ�����Ļ�ѧ����ʽ�� ��
���鰱����һ�ֻ�ѧ�����ǣ� ��
��ҩ���й��ġ��Ĵ�����֮һ���ڻ�ҩ�ڷ�����ըʱ���������µķ�Ӧ��2KNO3+C��S��K2S+2NO2��+CO2���������������� ����ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������̫�����屬�������������ˮ�ʵ����ض���Щ�¼��ٴ��������ҹ�ˮΣ���ľ��ӡ���̫������ij�������ŷŵ���ˮ�У�������ijЩ�������к������ʣ�������Ϊ���ܺ���Fe3����Ba2����K����OH����Cl���� ��
��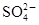 ��Ϊ�˽�һ��ȷ�ϣ�ȡ������ʵ���⣺
��Ϊ�˽�һ��ȷ�ϣ�ȡ������ʵ���⣺
��ȡ��ˮ��ϸ�۲죬����ɫ��������һ״̬��
����ȡ������ˮ�У�����ϡ���ᣬ�д�����ɫ�����������ټ�ϡ���ᣬ��ɫ��������ʧ��
����pH��ֽ�ⶨ��ˮ��pH����ֽ������ɫ��
(1)�ɴ˿�֪������ˮ�п϶����е�������___________���϶�û�е�������___________�����ܺ��е�������___________��
(2)���ͨ��ʵ���һ��ȷ����Һ�п��ܴ��ڵ�����__________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
д�����з�Ӧ�����ӷ�Ӧ����ʽ��ȫ�Ե�10�֣��д���Ϊ0�֣�
��1��NaAlO2��Һ�м�������������
��2��AlCl3��Һ�м��������NaOH
��3��Al2O3��NaOHˮ��Һ�ķ�Ӧ
��4��Fe3O4�ܽ���ϡ������
��5��FeCl2��Һ�м����ữ��H2O2��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һƿ�������Һ�����п��ܺ�H+��NH4+��K+��Cu2+��Fe3+��CO32-��I-�е�һ�ֻ��֣�ȡ����Һ��������ʵ�飺
����pH��ֽ���飬������Һ��ǿ����
��ȡ������Һ�������������Ƶ���ˮ��������CCl4����CCl4������ɫ
����ȡ������Һ����μ���ϡNaOH��Һ��ʹ��Һ��������ת��Ϊ���ԣ��ڵμӹ����м��μ���Ϻ���Һ�о���������
�ܽ��۵õ��ļ�����Һ���ȣ�������ų�����������ʹʪ��ĺ�ɫʯ����ֽ������
��������ʵ����ʵȷ�����ش�
��1������Һ�У��϶����ڵ��� ���϶������ڵ������� ��
��2������ȷ���Ƿ���ڵ�������_______��֤����(��)�Ƿ���ڵ�ʵ�鷽���� ��
��3��д��������漰�����ӷ���ʽ�����õ����ű������ת�Ƶķ������Ŀ ��
��4��д��������漰�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com