
Ėę×ÅĻÖ“ś¹¤ŅµµÄ·¢Õ¹£¬ÄÜŌ“ĪŹĢāŅŃ¾Ō½Ą“Ō½ŅżĘšČĖĆĒµÄÖŲŹÓ”£æĘѧ¼ŅĆĒŌ¤ŃŌ£¬Ī“Ą“×īĄķĻėµÄČ¼ĮĻŹĒĀĢÉ«Ö²Īļ£¬¼“½«Ö²ĪļµÄ½ÕøŃ(Ö÷ŅŖ³É·ÖŹĒĻĖĪ¬ĖŲ)ÓĆŹŹµ±µÄ“߻ƼĮĖ®½ā³ÉĘĻĢŃĢĒ£¬ŌŁ½«ĘĻĢŃĢĒ×Ŗ»Æ³ÉŅŅ“¼£¬ÓĆ×÷Č¼ĮĻ”£
(1)ŅŃÖŖ£ŗC2H5OH(l)£«3O2(g)ØD”ś2CO2(g)£«3H2O(l)
¦¤H£½£1367 kJ”¤mol£1
CH4(g)£«2O2(g)ØD”śCO2(g)£«2H2O(l)
¦¤H£½£890 kJ”¤mol£1
ČōijÖÖÖ²ĪļµÄ½ÕøŃŗ¬ĻĖĪ¬ĖŲŌ¼50%£¬ÓÉÖ²Īļ½ÕøŃ¾¹żŅ»ĻµĮŠ×Ŗ»ÆµĆµ½ŅŅ“¼ŌĮĻµÄ×ÜĄūÓĆĀŹĪŖ80%£¬ŌņÓĆ1000 g½ÕøŃĪŖŌĮĻÖʵƵÄŅŅ“¼Č¼ĮĻČ¼ÉÕĖł²śÉśµÄČČĮæÓė¶ąÉŁÉż¼×ĶéĶźČ«Č¼ÉÕ²śÉśµÄČČĮæĻąµ±(±ź×¼×“æöĻĀ)?
170L
”¾½āĪö”æ
ŹŌĢā·ÖĪö£ŗøĆĢāŹĒÓŠ¹ŲČČ»Æѧ·½³ĢŹ½µÄ¼ĘĖć£ŗ
Éč1000 g½ÕøŃÖʵƵÄŅŅ“¼Č¼ÉÕ²śÉśµÄČČĮæĪŖx£¬ÓėĢå»żĪŖyµÄ±ź×¼×“æöĻĀµÄ¼×ĶéĶźČ«Č¼ÉÕ²śÉśµÄČČĮæĻąµ±”£
(C6H10O5)n”«2nC2H5OH”«1367 kJ/mol”Į2n
162n g””””””””””””””””””””””2734n kJ
1000 g”Į50%”Į80%””””””””””””””x
½āµĆ£ŗx£½6750.6 kJ
CH4”””””«””””890 kJ/mol
22£®4 L””””””””890 kJ
””y””””””””””6750.6 kJ
½āµĆ£ŗy£½170 L”£
æ¼µć£ŗæ¼²éĻĖĪ¬ĖŲµÄŠŌÖŹ”¢·“Ó¦ČČµÄ¼ĘĖć
µćĘĄ£ŗøĆĢāŹĒÖŠµČÄѶȵďŌĢā£¬ŹŌĢā×¢ÖŲ»ł“”£¬²ąÖŲæ¼²éѧɜµÄ¹ę·¶“šĢāÄÜĮ¦”£øĆĢāÄŃ¶Č²»“ó£¬ÓŠĄūÓŚÅąŃųѧɜµÄĀß¼ĶĘĄķÄÜĮ¦£¬ŅŌ¼°·ÖĪöĪŹĢā”¢½ā¾öĪŹĢāµÄÄÜĮ¦”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
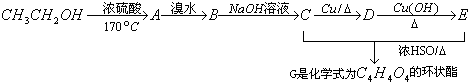
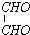
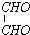
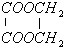
| H2O |
| H2O |
| ÅØH2SO4 |
| ”÷ |
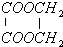 +2H2O
+2H2O| ÅØH2SO4 |
| ”÷ |
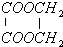 +2H2O
+2H2O²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| “߻ƼĮ |
| ”÷ |
| “߻ƼĮ |
| ”÷ |
| “߻ƼĮ |
| “߻ƼĮ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| “߻ƼĮ |
| ”÷ |
| “߻ƼĮ |
| ”÷ |
| µćČ¼ |
| µćČ¼ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø1£©Š“³öĀĢÉ«Ö²Īļ½ÕøŃ×Ŗ»ÆĪŖŅŅ“¼µÄ»Æѧ·½³ĢŹ½£ŗ
¢Ł___________________________________£¬¢Ś______________________________________”£
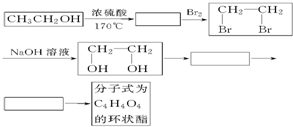
£Ø2£©ŅŅ“¼³żÓĆ×÷Č¼ĮĻĶā£¬»¹æÉŅŌÓĆĖüŗĻ³ÉĘäĖūÓŠ»śĪļ”£ĻĀĮŠÖ÷ŅŖŹĒŅŌŅŅ“¼ĪŖĘšŹ¼ŌĮĻµÄ×Ŗ»Æ¹ŲĻµĶ¼£¬ĒėŌŚ±ķÖŠĢīÉĻĻąÓ¦ĪļÖŹµÄ½į¹¹¼ņŹ½”£
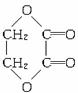
£Ø3£©Š“³öÉĻŹö×Ŗ»Æ¹ŲĻµĶ¼ÓÉ
CH2OHCH2OH![]() C4H4O4µÄ»Æѧ·½³ĢŹ½£ØÓŠ»śĪļÓĆ½į¹¹¼ņŹ½±ķŹ¾£©”£
C4H4O4µÄ»Æѧ·½³ĢŹ½£ØÓŠ»śĪļÓĆ½į¹¹¼ņŹ½±ķŹ¾£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ÄźŠĀ½®ĪŚĀ³Ä¾ĘėŹŠøßŅ»ĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ»Æѧ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø10·Ö£©£®Ėę×ÅĻÖ“ś¹¤ŅµµÄ·¢Õ¹£¬ÄÜŌ“ĪŹĢāŅŃ¾Ō½Ą“Ō½ŅżĘšČĖĆĒµÄÖŲŹÓ£¬æĘѧ¼ŅŌ¤ŃŌ£¬Ī“Ą“×īĄķĻėµÄČ¼ĮĻŹĒĀĢÉ«Ö²Īļ£¬¼“½«Ö²ĪļµÄ½ÕøŃ(Ö÷ŅŖ³É·ÖŹĒĻĖĪ¬ĖŲ)ÓĆŹŹµ±µÄ“߻ƼĮæÉŅŌĖ®½ā³ÉĘĻĢŃĢĒ£¬ŌŁ½«ĘĻĢŃĢĒ×Ŗ»ÆĪŖŅŅ“¼£¬ÓĆ×öČ¼ĮĻ”£
(1)Š“³öĀĢÉ«Ö²ĪļµÄ½ÕøŃ×Ŗ»ÆĪŖŅŅ“¼µÄ»Æѧ·½³ĢŹ½£ŗ
¢Ł____________________________________
¢Ś________________________________________”£
(2)ŅŅ“¼³żÓĆ×öČ¼ĮĻĶā£¬»¹æÉŅŌÓĆĖüŗĻ³ÉĘäĖūÓŠ»śĪļ£¬ŅŌŅŅ“¼ĪŖĘšŹ¼ŌĮĻµÄ×Ŗ»Æ¹ŲĻµČēĶ¼ĖłŹ¾£ŗ£ØŅŃÖŖ;ŌŚÓŠ“߻ƼĮµÄĢõ¼žĻĀ£¬¶ą²½Ńõ»ÆR-CH2OH”śR-CHO”śRCOOH£©
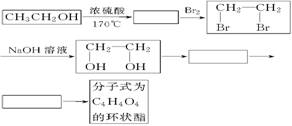
ĒėŌŚ·½æņÖŠĢīÉĻĻąÓ¦ĪļÖŹµÄ½į¹¹¼ņŹ½”£
(3)Š“³öÉĻĆę×Ŗ»Æ¹ŲĻµĶ¼ÖŠÓÉCH2OHCH2OHØD”śC4H4O4µÄ»Æѧ·½³ĢŹ½(ÓŠ»śĪļÓĆ½į¹¹¼ņŹ½±ķŹ¾)”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com