
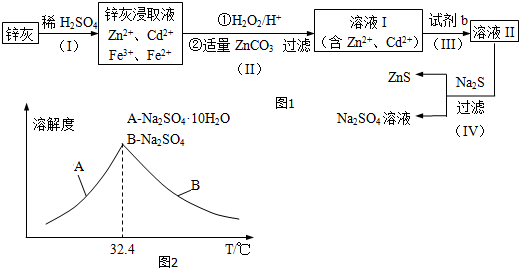
���� п�������ᷴӦ�ú��������ӡ�п���ӡ������ӡ��������ӵȵ���Һ������˫��ˮ����������������Ϊ���������ӣ���̼��п����ƽ��pHʹFe��OH��3��ȫ���������˺�õ����������ӡ�п���ӵ���Һ�������Fe��OH��3��ZnCO3��������пɵ�Cd���ʣ�Ϊ���������µ����ʣ��Լ�bӦΪп�����˺����Һ��Ϊ����п��Һ������п��Һ�м������ƿɵ������ƺ���п��
��1��������Ч��ѧ��Ӧ���ʵ����ط���������衢�ʵ����¡���������ϸ�ɷ�ĩ�����衢��ν�ȡ�ȶ������п�ҵĽ�ȡ�ʣ�
��2����������ķ����жϲ����������������ɣ�
��3�����ݷ�����֪�Լ�b�����ƣ�
��4����ZnS�����Ǵ���������Һ�������ģ����Լ���ZnS�����Ƿ�ϴ�Ӹɾ�������ͨ������ϴ��Һ���Ƿ�������������жϣ�
�ڵ����ʵ�����Na2S04��CH4�ڸ��¡���������������Na2S������Ԫ���غ�д���û�ѧ��Ӧ����ʽ��
�۸����ܽ�����¶ȱ仯���߷�������Һ�еõ�Na2SO4.10H2O�IJ���������
��5�����������CdΪd mol���������û��ӵ�п�����ʵ���Ϊd mol�������Ƶ����ʵ���Ϊ��VL��c mol/L=cVmol����������п�����ʵ���ΪcVmol������пԪ���غ��֪����Ʒ��пԪ�ص����ʵ�����Ȼ�����m=nM�����п���к���пԪ�ص�������
��� �⣺п�������ᷴӦ�ú��������ӡ�п���ӡ������ӡ��������ӵȵ���Һ������˫��ˮ����������������Ϊ���������ӣ���̼��п����ƽ��pHʹFe��OH��3��ȫ���������˺�õ����������ӡ�п���ӵ���Һ�������Fe��OH��3��ZnCO3��������пɵ�Cd���ʣ�Ϊ���������µ����ʣ��Լ�bӦΪп�����˺����Һ��Ϊ����п��Һ������п��Һ�м������ƿɵ������ƺ���п��
��1�����衢�ʵ����¡���������ϸ�ɷ�ĩ�����衢��ν�ȡ�ȶ������п�ҵĽ�ȡ�ʣ������õķ����Ǣݼ�ѹ��
�ʴ�Ϊ���ݣ�
��2����������ķ�����֪���������������ΪFe��OH��3��ZnCO3��
�ʴ�Ϊ��Fe��OH��3��ZnCO3��
��3����������ķ�����֪���Լ�bӦΪп��
�ʴ�Ϊ��Zn����п����
��4����ZnS�����Ǵ���������Һ�������ģ����Լ���ZnS�����Ƿ�ϴ�Ӹɾ��ķ�����ȡ���һ��ϴ��Һ�������Թܣ��μӼ���BaCl2��Һ�������ֻ�����δϴ������֮����ϴ����
�ʴ�Ϊ��ȡ���һ��ϴ��Һ�������Թܣ��μӼ���BaCl2��Һ�������ֻ�����δϴ������֮����ϴ����
�ڵ����ʵ�����Na2S04��CH4�ڸ��¡���������������Na2S������Ԫ���غ��֪���û�ѧ��Ӧ����ʽΪ��Na2S04+CH4$\frac{\underline{\;\;\;����\;\;\;}}{����}$Na2S+2H2O+CO2��
�ʴ�Ϊ��Na2S04+CH4$\frac{\underline{\;\;\;����\;\;\;}}{����}$Na2S+2H2O+CO2��
�۸����ܽ�����¶ȱ仯���߿�֪������Һ�еõ�Na2SO4.10H2O�IJ�������������Ũ�������½ᾧ�����ˣ�
�ʴ�Ϊ������Ũ�������½ᾧ�����ˣ�
��5�����������CdΪd mol���������û��ӵ�п�����ʵ���Ϊd mol�������Ƶ����ʵ���Ϊ��VL��c mol/L=cVmol����������п�����ʵ���ΪcVmol��
����пԪ���غ��֪����Ʒ��пԪ�ص����ʵ���Ϊ��cVmol-d mol-b mol��
����п���к���пԪ�ص�����Ϊ��65g/mol����cVmol-d mol-b mol��=65��Vc-b-d��g��
�ʴ�Ϊ��65��Vc-b-d��g��
���� ����Ϊ���������⣬ͨ���ӷ�������ȡ��п�Ĺ������̣��������ܽ�ƽ����ƶ���������ԭ����ʽ����д�����ʵķ����֪ʶ����Ϥ���ʵ����ʡ���ȷ�����ǽ���ؼ����������ѧ���������⡢���������������������Ŀ�Ѷ��еȣ�


 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д� ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
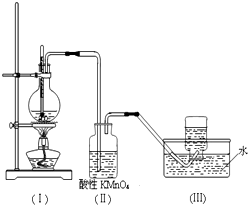 ��ͼ��ʾ����ʵ�����ͨ������Ũ�������Ҵ���ϼ�������ϩ������һ��ʱ���۲쵽��ƿ����Һ��ڣ�װ�ã����о������ữ�ĸ��������Һ��ɫ��
��ͼ��ʾ����ʵ�����ͨ������Ũ�������Ҵ���ϼ�������ϩ������һ��ʱ���۲쵽��ƿ����Һ��ڣ�װ�ã����о������ữ�ĸ��������Һ��ɫ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
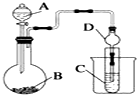 ij��ѧ��ȤС��Ϊ̽��Ԫ�����ʵĵݱ���ɣ����������ϵ��ʵ�飮
ij��ѧ��ȤС��Ϊ̽��Ԫ�����ʵĵݱ���ɣ����������ϵ��ʵ�飮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
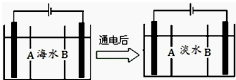 ������Ա��ij����ɽȪˮ���з������飬�����ʾɽȪˮ����Ӳˮ��
������Ա��ij����ɽȪˮ���з������飬�����ʾɽȪˮ����Ӳˮ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
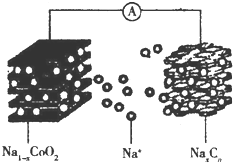 ��ҡ���͡������ӵ�س�ŵ�ԭ��ΪNaCoO2+Cn$?_{���}^{�ŵ�}$Na1-xCoO2+NaxCn����ؽṹ��ͼ��ʾ������˵����ȷ���ǣ�������
��ҡ���͡������ӵ�س�ŵ�ԭ��ΪNaCoO2+Cn$?_{���}^{�ŵ�}$Na1-xCoO2+NaxCn����ؽṹ��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | �ŵ�ʱ��Na+���ƶ� | |
| B�� | �ŵ�ʱ���ܵĻ��ϼ����� | |
| C�� | ���ʱ��������������С | |
| D�� | ���ʱ�������ĵ缫��ӦʽΪNaCoO2-xe-�TNa1-xCoO2+xNa+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ȡij������Һ����������KSCN��Һ�����������ٵ��뼸����ˮ������Һ��Ѫ��ɫ��˵��ԭ�����к�Fe2+ | |
| B�� | ������Һ��ϡ���Ṳ�ȺӼ��кͣ��ټ�����������Һˮԡ���ȣ��й������������� | |
| C�� | ����������Ƿ������ȩ��������Ʒ�м�������NaOH��Һ���кͼ��ᣬ����������Ӧ��������Cu��OH��2���ȵ�ʵ�� | |
| D�� | Һ̬�������м���ϡNaOH��Һ���м����ӣ�Ȼ���������ϡ���ᣬ�ټ���AgNO3��Һ�������������Ƿ�����Ԫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
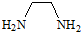 ����һ�������·�����Ӧ����1mol EDTA��4mol HCl����EDTA�ķ���ʽΪ��������
����һ�������·�����Ӧ����1mol EDTA��4mol HCl����EDTA�ķ���ʽΪ��������| A�� | C10H16N2O8 | B�� | C10H20N2O8 | C�� | C8H16N2O8 | D�� | C16H20N2O8Cl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com