
���� ��1��ȡ�������壬����������ˮ����������ȫ���ܽ⣬�õ���ɫ����Һ��������ɫ������һ�������ڣ�������Cu2+���������γɵ���������ˮ��
��2����1���γɵ���Һ�м���NaOH��Һ�����Ա仯����һ������Mg2+�����ȣ�û�������������һ������NH4+���ۺϣ�1����2����֪��һ������K+��
��3����ȡ�������壬����ϡ���ᣬ��������ȫ���ܽ⣬������ų�����һ������CO32-������Һ�к���Cl-��SO42-��
��� �⣺��1��ȡ�������壬����������ˮ����������ȫ���ܽ⣬�õ���ɫ����Һ��������ɫ������һ�������ڣ�������Cu2+���������γɵ���������ˮ��
��2����1���γɵ���Һ�м���NaOH��Һ�����Ա仯����һ������Mg2+�����ȣ�û�������������һ������NH4+���ۺϣ�1����2����֪��һ������K+��
��3����ȡ�������壬����ϡ���ᣬ��������ȫ���ܽ⣬������ų�����һ������CO32-������Һ�к���Cl-��SO42-��
������������֪�������ĩ��һ�������е�������NH4+��Cu2+��Mg2+��CO32-��һ������K+��Cl-��SO42-�е�����һ�֣��ʴ�Ϊ��NH4+��Cu2+��Mg2+��CO32-��K+��Cl-��SO42-�е�����һ�֣�
���� ���⿼�鳣�����ӵļ��飬�Ѷ��еȣ�����ע��ͨʵ�������жϣ��������ʵĵ������ʣ���Ϊ�ƶϵ�ͻ�ƿڣ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ø�������̼��Ʒ�Ӧ��ȡ������̼��֤����Ԫ�طǽ����Դ���̼Ԫ�� | |
| B�� | ���ࡢ��֬����������һ�������¶��ܷ���ˮ�ⷴӦ | |
| C�� | �赥���ڵ��ӹ�ҵ��Ӧ�ù㷺���������ά | |
| D�� | ʯ�͵��ѻ��������塢ֲ�����Լ����������ʹ���Ը��������Һ��ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���������������������һ����ͬ | |
| B�� | ���������������������һ����ͬ | |
| C�� | Y������CH4��O2��N2������֮�ȿ���Ϊ1��6��3 | |
| D�� | Y������CH4��O2��N2�����ʵ�����֮��һ��Ϊ1��3��6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
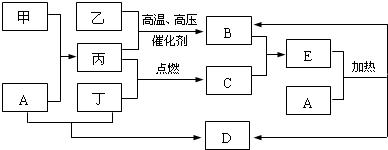
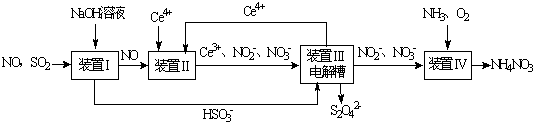
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����£�pH=1����Һ�У�Na+��Fe3+��NO3-��I- | |
| B�� | ˮ�����H+Ũ��Ϊ1��10-13mol•L-1����Һ�У�K+��Al3+��Cl-��SO42- | |
| C�� | AlO2?Ũ��Ϊ0.1 mol•L-1����Һ�У�Na+��K+��HCO3-��Cl- | |
| D�� | ����KSCN��Һ�Ժ�ɫ����Һ��K+��NH4+��Cl-��NO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
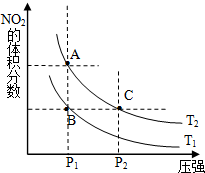 ��1���Է�ӦN2O4��g���T2NO2��g����H��0�����¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ��T1��T2�����������������=������A��C���������VA��VC��ͬ�ϣ���
��1���Է�ӦN2O4��g���T2NO2��g����H��0�����¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ��T1��T2�����������������=������A��C���������VA��VC��ͬ�ϣ���| ʱ�䣨s�� | 0 | 20 | 40 | 60 | 80 |
| n��NO2��/mol | 0.40 | n1 | 0.26 | n3 | n4 |
| n��N2O4��/mol | 0.00 | 0.05 | n2 | 0.08 | 0.08 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
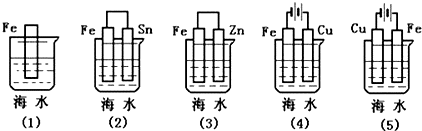
| A�� | ��5����2����1����3����4�� | B�� | ��5����2����3����1����4�� | C�� | ��4����2����1����3����5�� | D�� | ��4����2����1����5����3�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com